1.3 Introduction to Biological Macromolecules
2 min read•november 18, 2024
Danna Esther Gelfand
Haseung Jun
Danna Esther Gelfand
Haseung Jun
Skills you’ll gain in this topic:
- Describe the relationship between monomers and polymers in biological macromolecules.
- Illustrate dehydration synthesis and hydrolysis in building and breaking down macromolecules.
- Define the basic structures and functions of carbohydrates, lipids, proteins, and nucleic acids.
- Recognize the monomers of each macromolecule and their biological roles.
- Connect macromolecule structure to its function in cells.
Chemical Bonds
Covalent: Sharing of electrons (Molecule created by two or more atoms in a covalent bond). A single bond (sharing one pair of electrons) in a structural formula is represented by one line connecting two atoms. A double bond is the sharing of two pairs of electrons. A structural formula is represented by two lines connecting two atoms. Electronegativity is the atom's attraction for electrons in a covalent bond.
Examples: methane (CH4), carbon monoxide (CO), and iodine monobromide (IBr).
Ionic: Transfer of electrons/electrostatic attraction between a positive and negative ion (after the transfer, both atoms have complete valence shells) (ionic compounds/salts formed).
Examples: Sodium Chloride NaCl, Lithium Fluoride LiF.
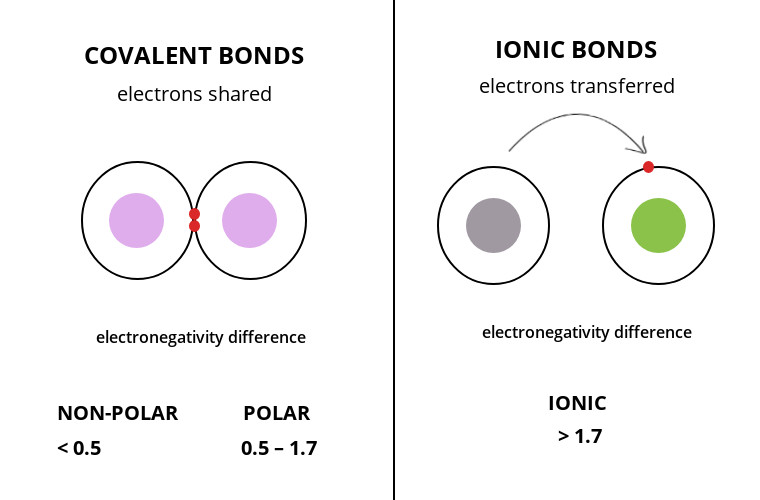
Image courtesy of Surfguppy.
Metallic Bonds
Metallic bonds are formed by the attraction between metal ions and delocalized, or "free", electrons. Examples: iron, cobalt, silver, gold, platinum, copper, zinc.
Polymers and Monomers
Polymer: a long molecule composed of many molecules bonded together covalently. (composed of multiple monomers) (Poly-many). Out of the main biological macromolecules, lipids, do not usually form polymers.
Monomer: are small building block molecules that, when combined, make a polymer. (mono-one)
Covalent Bonds
Nonpolar Covalent bond: the equal sharing of electrons and distribution of charge (smaller electronegativity differences as seen in the figure above).
Polar covalent bond: Unequal sharing of electrons and distribution of charge causes partial positive or partial negative for each atom or molecule (higher electronegativity differences as seen in the image above).
- InTRAmolecular bonds: within the molecule (Covalent bonds).
- InTERmolecular bonds: between water molecules (Hydrogen bonds). Hydrogen atom bonded with an electronegative atom is attracted to another electronegative atom. The ability of hydrogen to interact with Fluorine, Nitrogen, and oxygen (FNO). (WEAK ATTRACTION).
It is important to note that Hydrogen bonds are NOT covalent bonds.
Dehydration Synthesis & Hydrolysis
Dehydration Synthesis: occurs when monomers combine to form a polymer through a reaction after water is removed (dehydrate - water lost). One monomer donates OH- and another monomer donates H+ forming H20. It is a condensation reaction, requires energy (making it an endergonic reaction) and enzymes, and builds complexity (anabolic- small molecules bind together to form larger molecules).
Hydrolysis: occurs when polymers are broken down into monomers through a reaction due to the addition of water (hydro - water, lysis - break) (Digestion). It uses H2O to break down the molecules splitting into H+ and OH-. Releases energy (exergonic) and requires enzymes. Reduces complexity (catabolic).
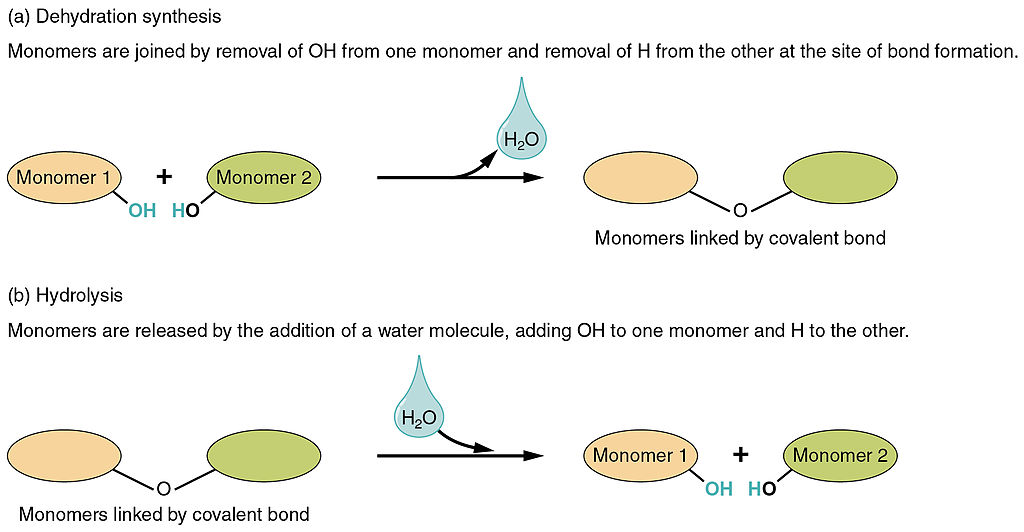
Image courtesy of WikiMedia Commons.
<< Hide Menu
1.3 Introduction to Biological Macromolecules
2 min read•november 18, 2024
Danna Esther Gelfand
Haseung Jun
Danna Esther Gelfand
Haseung Jun
Skills you’ll gain in this topic:
- Describe the relationship between monomers and polymers in biological macromolecules.
- Illustrate dehydration synthesis and hydrolysis in building and breaking down macromolecules.
- Define the basic structures and functions of carbohydrates, lipids, proteins, and nucleic acids.
- Recognize the monomers of each macromolecule and their biological roles.
- Connect macromolecule structure to its function in cells.
Chemical Bonds
Covalent: Sharing of electrons (Molecule created by two or more atoms in a covalent bond). A single bond (sharing one pair of electrons) in a structural formula is represented by one line connecting two atoms. A double bond is the sharing of two pairs of electrons. A structural formula is represented by two lines connecting two atoms. Electronegativity is the atom's attraction for electrons in a covalent bond.
Examples: methane (CH4), carbon monoxide (CO), and iodine monobromide (IBr).
Ionic: Transfer of electrons/electrostatic attraction between a positive and negative ion (after the transfer, both atoms have complete valence shells) (ionic compounds/salts formed).
Examples: Sodium Chloride NaCl, Lithium Fluoride LiF.

Image courtesy of Surfguppy.
Metallic Bonds
Metallic bonds are formed by the attraction between metal ions and delocalized, or "free", electrons. Examples: iron, cobalt, silver, gold, platinum, copper, zinc.
Polymers and Monomers
Polymer: a long molecule composed of many molecules bonded together covalently. (composed of multiple monomers) (Poly-many). Out of the main biological macromolecules, lipids, do not usually form polymers.
Monomer: are small building block molecules that, when combined, make a polymer. (mono-one)
Covalent Bonds
Nonpolar Covalent bond: the equal sharing of electrons and distribution of charge (smaller electronegativity differences as seen in the figure above).
Polar covalent bond: Unequal sharing of electrons and distribution of charge causes partial positive or partial negative for each atom or molecule (higher electronegativity differences as seen in the image above).
- InTRAmolecular bonds: within the molecule (Covalent bonds).
- InTERmolecular bonds: between water molecules (Hydrogen bonds). Hydrogen atom bonded with an electronegative atom is attracted to another electronegative atom. The ability of hydrogen to interact with Fluorine, Nitrogen, and oxygen (FNO). (WEAK ATTRACTION).
It is important to note that Hydrogen bonds are NOT covalent bonds.
Dehydration Synthesis & Hydrolysis
Dehydration Synthesis: occurs when monomers combine to form a polymer through a reaction after water is removed (dehydrate - water lost). One monomer donates OH- and another monomer donates H+ forming H20. It is a condensation reaction, requires energy (making it an endergonic reaction) and enzymes, and builds complexity (anabolic- small molecules bind together to form larger molecules).
Hydrolysis: occurs when polymers are broken down into monomers through a reaction due to the addition of water (hydro - water, lysis - break) (Digestion). It uses H2O to break down the molecules splitting into H+ and OH-. Releases energy (exergonic) and requires enzymes. Reduces complexity (catabolic).

Image courtesy of WikiMedia Commons.

© 2024 Fiveable Inc. All rights reserved.