1.4 Properties of Biological Macromolecules
4 min read•november 18, 2024
Danna Esther Gelfand
Haseung Jun
Danna Esther Gelfand
Haseung Jun
Skills you’ll gain in this topic:
- Analyze how carbohydrates, lipids, proteins, and nucleic acids’ structures determine their functions.
- Explain how molecular variations impact roles like energy storage and membrane structure.
- Distinguish the types of bonds that stabilize macromolecule structures.
- Relate protein structure levels (primary, secondary, tertiary, quaternary) to their functions.
- Demonstrate how macromolecular interactions support cellular processes.
How Structure Impacts Function in Macromolecules
Macromolecules are large molecules composed of two or more polymers combined together (macro=large).
Carbohydrates
Carbohydrates are sugars and polymers of sugars (usually ends in the suffix -ose). There are multiple hydroxyl groups and a carbonyl group. If the carbonyl group is at an end, the sugar has an aldehyde and is known as an aldose sugar. If the carbonyl group is in the middle, the sugar has a ketone so it is a ketose sugar.
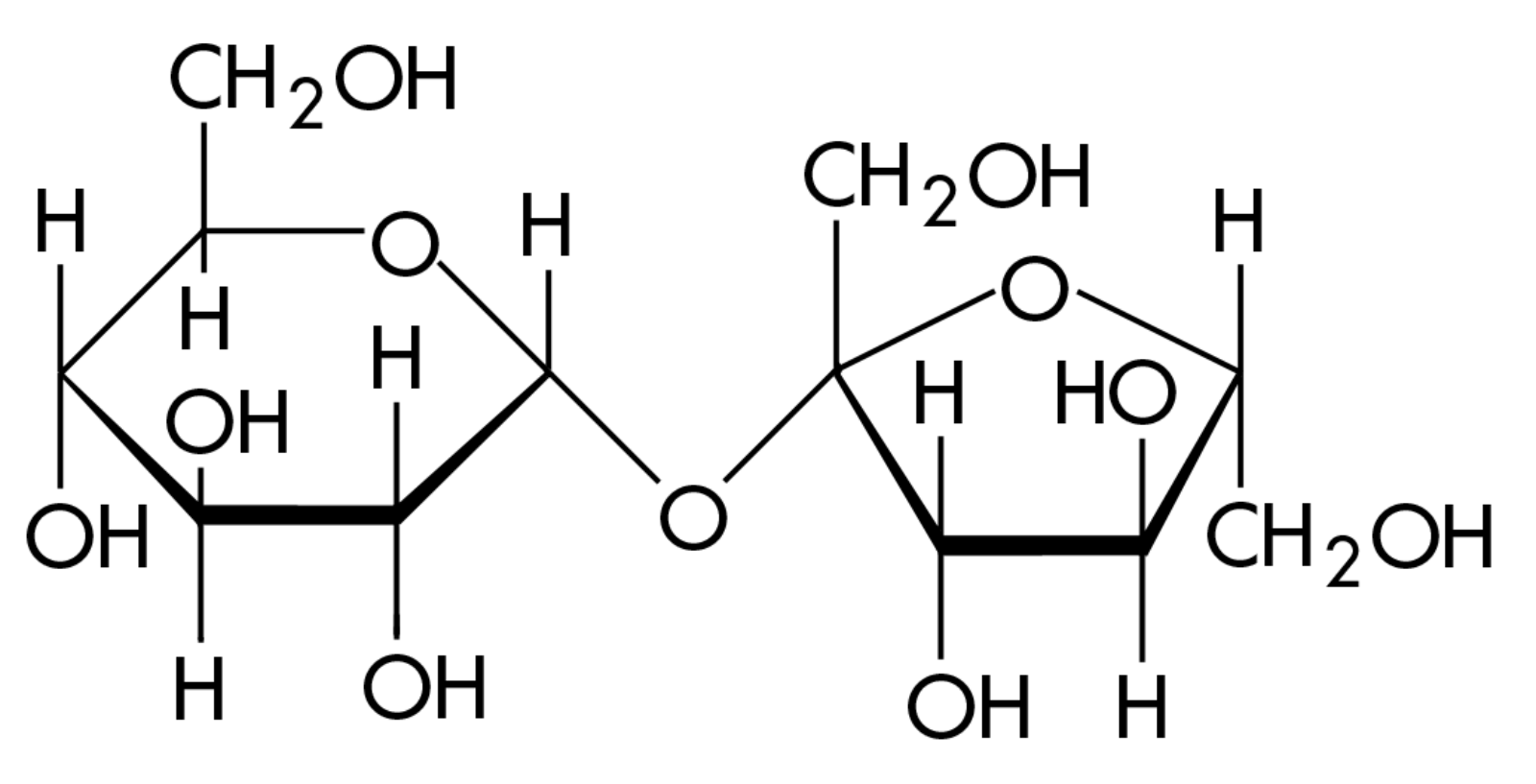
Image courtesy of WikiMedia Commons.
Monosaccharide – simple sugar – generally has a formula that is a multiple of the unit CH 2 O, 1:2:1 ratio. Glucose (C 6 H 12 O 6 ), is the most common monosaccharide. (important in glycolysis) (mono-one) Another common example is fructose.
You'll see this is a pattern with macromolecules, but its structure determines its function, and in the case of carbohydrates, this will hold true!
Lipids
Lipids are the only class of macromolecules that DO NOT form polymers. Non-polar molecules and hydrophobic- no affinity for water (avoids water). Mostly hydrocarbons. Important storage molecule. Most energy-rich (9 calories per gram) Important for insulation in animals. Fats are made up of glycerol and three fatty acids joined by an ester bond/linkage forming a triglyceride/triacylglycerol. Separate from water.
Saturated and Unsaturated Fatty Acids
Glycerol is a three-carbon alcohol with a hydroxyl group attached to each carbon. Fatty Acids are long chains of carbon attached to a carboxyl group and can be saturated or unsaturated. Saturated Fatty Acids have no double bonds between carbon atoms. contain a maximum number of hydrogen atoms, usually found in animals and solidify at room temperature (examples include lard and butter) Unsaturated Fatty Acids have one or more double and/or triple bonds between carbon atoms and are liquids at room temperature (examples are typically from plants, fish, and vegetable oils).
Phospholipids
Phospholipids are one of the fatty acids of a triglyceride, replaced by a phosphate group. 1 glycerol, 2 fatty acids, and 1 phosphate group. A major component of the cell membrane called the phospholipid bilayer. The head is hydrophilic (attracts water) and contains the glycerol and phosphate group. The tail is hydrophobic (avoids water) and contains two fatty acids, one is unsaturated and the other is saturated). Phospholipid assembles into the bilayer found in cell membranes when added to water.
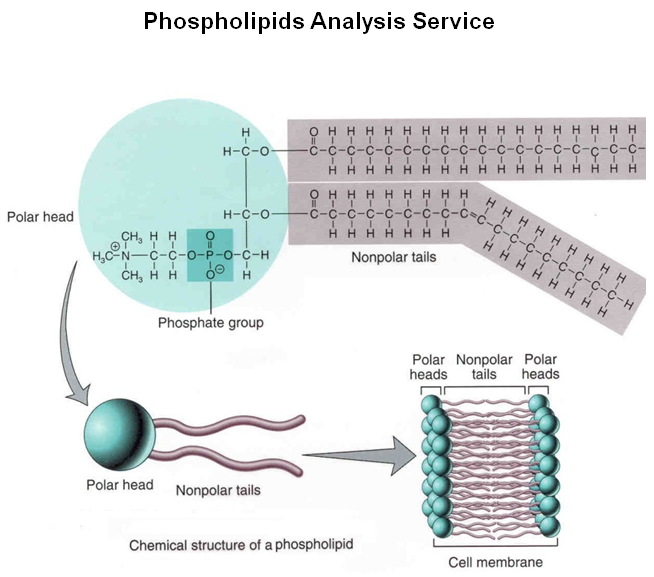
Image courtesy of Creative Proteomics.
Steroids
A steroid is a carbon skeleton made up of 4 fused rings. Although they have different structures than most lipids, steroids still classify as lipids because they are hydrophobic and insoluble in water. Every steroid has 4 linked carbon rings and many have short tails. Cholesterol is a type of steroid with a short tail, as well as a hydroxide functional group attached to it. This steroid is an important component of animal cell membranes. Any steroid with a hydroxide functional group is also classified as an alcohol, thus why they are called sterols.
Proteins
Proteins are made of 20 different monomer amino acids joined by peptide bonds (also called “polypeptides”) which are covalent bonds. The structure of the amino acids have specified chemical and physical properties that determine their function so a slight change in structure at the primary stage can lead to the change in both a protein's structure and function. (4 calories per gram)
When two amino acids are joined together by dehydration synthesis, it is called a dipeptide (di-two). Polymers of amino acids are polypeptides and are formed when many amino acids join through dehydration synthesis. The folding of one or more polypeptides forms a protein.
Some amino acids are polar and other are nonpolar; in addition, some are acidic and others basic. This directly impacts the protein in many ways because it affects the protein's shape and therefore its function 🦾. Because of the physical properties of each amino acid, the folding will differ (polar attracts polar, acid attracts acid), and one small change in the peptide chain can make a huge impact.
Each amino acid consists of an amino group, carboxyl group and R group (with chemical properties of hydrophobic, hydrophilic, and ionic). It's actually these R groups that determine the property of the amino acid and therefore the folding in the long run. When you encounter a question about polarity of an amino acid, take a look at its R group - it should tell you the answer!
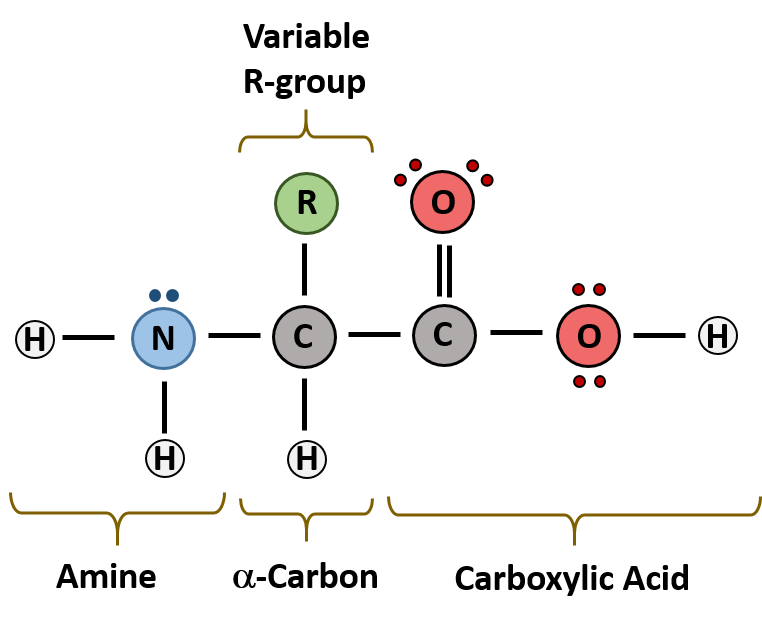
Image Courtesy of Western Oregon University
Nucleic Acids
Nucleic acids contain carbon, hydrogen, oxygen and nitrogen, but it also consists of phosphurus. Made up of monomers called nucleotides, nucleic acids make up the genetic information, or our DNA. Nucleotides consists of a five-carbon sugar, a phosphate, and a nitrogen base (adenine, thymine, guanine, cytosine, uracil).
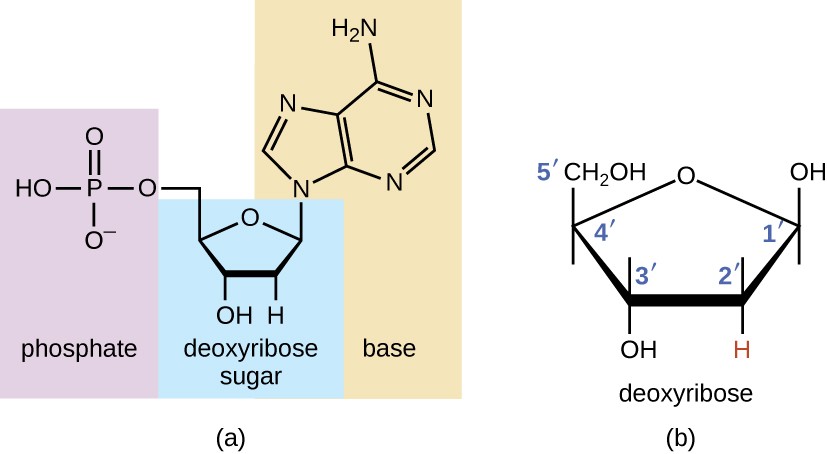
Image Courtesy of Lumen Learning
Nucleic acids also make up our RNA, which is a little different from DNA (you'll learn more in later units!)
Big Fat Summary
Macromolecules are THE CORE of life, so it might be (more than) useful to know and have them memorized! If you're still confused with some things, here's a nice table to summarize!
| Macromolecule | Monomer | Polymer | Linkage Bond | Elements |
| Carbohydrates | monosaccharide | polysaccharide | glycosidic | C,H,O |
| Proteins | amino acid | polypeptide | peptide bond | C,H,O,N,S |
| Nucleic Acids | nucleotide | DNA, RNA | sugar-phosphate bond | C,H,O,N,P |
| Lipids | disclaimer is not really a polymer, just a long chain of hydrogens and carbons | phospholipids | ester bonds | C,H,O,P |
<< Hide Menu
1.4 Properties of Biological Macromolecules
4 min read•november 18, 2024
Danna Esther Gelfand
Haseung Jun
Danna Esther Gelfand
Haseung Jun
Skills you’ll gain in this topic:
- Analyze how carbohydrates, lipids, proteins, and nucleic acids’ structures determine their functions.
- Explain how molecular variations impact roles like energy storage and membrane structure.
- Distinguish the types of bonds that stabilize macromolecule structures.
- Relate protein structure levels (primary, secondary, tertiary, quaternary) to their functions.
- Demonstrate how macromolecular interactions support cellular processes.
How Structure Impacts Function in Macromolecules
Macromolecules are large molecules composed of two or more polymers combined together (macro=large).
Carbohydrates
Carbohydrates are sugars and polymers of sugars (usually ends in the suffix -ose). There are multiple hydroxyl groups and a carbonyl group. If the carbonyl group is at an end, the sugar has an aldehyde and is known as an aldose sugar. If the carbonyl group is in the middle, the sugar has a ketone so it is a ketose sugar.

Image courtesy of WikiMedia Commons.
Monosaccharide – simple sugar – generally has a formula that is a multiple of the unit CH 2 O, 1:2:1 ratio. Glucose (C 6 H 12 O 6 ), is the most common monosaccharide. (important in glycolysis) (mono-one) Another common example is fructose.
You'll see this is a pattern with macromolecules, but its structure determines its function, and in the case of carbohydrates, this will hold true!
Lipids
Lipids are the only class of macromolecules that DO NOT form polymers. Non-polar molecules and hydrophobic- no affinity for water (avoids water). Mostly hydrocarbons. Important storage molecule. Most energy-rich (9 calories per gram) Important for insulation in animals. Fats are made up of glycerol and three fatty acids joined by an ester bond/linkage forming a triglyceride/triacylglycerol. Separate from water.
Saturated and Unsaturated Fatty Acids
Glycerol is a three-carbon alcohol with a hydroxyl group attached to each carbon. Fatty Acids are long chains of carbon attached to a carboxyl group and can be saturated or unsaturated. Saturated Fatty Acids have no double bonds between carbon atoms. contain a maximum number of hydrogen atoms, usually found in animals and solidify at room temperature (examples include lard and butter) Unsaturated Fatty Acids have one or more double and/or triple bonds between carbon atoms and are liquids at room temperature (examples are typically from plants, fish, and vegetable oils).
Phospholipids
Phospholipids are one of the fatty acids of a triglyceride, replaced by a phosphate group. 1 glycerol, 2 fatty acids, and 1 phosphate group. A major component of the cell membrane called the phospholipid bilayer. The head is hydrophilic (attracts water) and contains the glycerol and phosphate group. The tail is hydrophobic (avoids water) and contains two fatty acids, one is unsaturated and the other is saturated). Phospholipid assembles into the bilayer found in cell membranes when added to water.

Image courtesy of Creative Proteomics.
Steroids
A steroid is a carbon skeleton made up of 4 fused rings. Although they have different structures than most lipids, steroids still classify as lipids because they are hydrophobic and insoluble in water. Every steroid has 4 linked carbon rings and many have short tails. Cholesterol is a type of steroid with a short tail, as well as a hydroxide functional group attached to it. This steroid is an important component of animal cell membranes. Any steroid with a hydroxide functional group is also classified as an alcohol, thus why they are called sterols.
Proteins
Proteins are made of 20 different monomer amino acids joined by peptide bonds (also called “polypeptides”) which are covalent bonds. The structure of the amino acids have specified chemical and physical properties that determine their function so a slight change in structure at the primary stage can lead to the change in both a protein's structure and function. (4 calories per gram)
When two amino acids are joined together by dehydration synthesis, it is called a dipeptide (di-two). Polymers of amino acids are polypeptides and are formed when many amino acids join through dehydration synthesis. The folding of one or more polypeptides forms a protein.
Some amino acids are polar and other are nonpolar; in addition, some are acidic and others basic. This directly impacts the protein in many ways because it affects the protein's shape and therefore its function 🦾. Because of the physical properties of each amino acid, the folding will differ (polar attracts polar, acid attracts acid), and one small change in the peptide chain can make a huge impact.
Each amino acid consists of an amino group, carboxyl group and R group (with chemical properties of hydrophobic, hydrophilic, and ionic). It's actually these R groups that determine the property of the amino acid and therefore the folding in the long run. When you encounter a question about polarity of an amino acid, take a look at its R group - it should tell you the answer!

Image Courtesy of Western Oregon University
Nucleic Acids
Nucleic acids contain carbon, hydrogen, oxygen and nitrogen, but it also consists of phosphurus. Made up of monomers called nucleotides, nucleic acids make up the genetic information, or our DNA. Nucleotides consists of a five-carbon sugar, a phosphate, and a nitrogen base (adenine, thymine, guanine, cytosine, uracil).

Image Courtesy of Lumen Learning
Nucleic acids also make up our RNA, which is a little different from DNA (you'll learn more in later units!)
Big Fat Summary
Macromolecules are THE CORE of life, so it might be (more than) useful to know and have them memorized! If you're still confused with some things, here's a nice table to summarize!
| Macromolecule | Monomer | Polymer | Linkage Bond | Elements |
| Carbohydrates | monosaccharide | polysaccharide | glycosidic | C,H,O |
| Proteins | amino acid | polypeptide | peptide bond | C,H,O,N,S |
| Nucleic Acids | nucleotide | DNA, RNA | sugar-phosphate bond | C,H,O,N,P |
| Lipids | disclaimer is not really a polymer, just a long chain of hydrogens and carbons | phospholipids | ester bonds | C,H,O,P |

© 2024 Fiveable Inc. All rights reserved.