1.1 Structure of Water and Hydrogen Bonding
4 min read•november 18, 2024
Danna Esther Gelfand
Jillian Holbrook
Danna Esther Gelfand
Jillian Holbrook
Skills you’ll gain in this topic:
- Understand how water’s polarity and hydrogen bonds create unique properties.
- Discuss how cohesion, adhesion, and surface tension support biological processes.
- Illustrate examples of water’s high specific heat and temperature stability effects.
- Analyze how water’s role as a solvent supports life at the molecular level.
- Connect water’s properties to its essential role in supporting organisms.
Water Molecules 💧
Water is a polar molecule, meaning the ends have opposite partial charges or unequal distribution of charge. The chemical formula of water H2O tells us that a water molecule is made up of two hydrogens and one oxygen. Water is polar because the hydrogens carry partial positive charges, and oxygen has a partial negative charge, which we can examine further when we discuss hydrogen bonding.
The simple phrase, "like attracts like," helps us to remember that polar substances are attracted to other polar substances, just as nonpolar substances are attracted to other nonpolar substances. Ever tried mixing oil and water? Oil molecules are nonpolar and have equally balanced charges rather than positive and negative poles like water. Because of this, oil and water are immiscible or do not combine. Polar and nonpolar do not attract because they are not alike.
In regard to water, there is specific terminology to describe attractive interactions. A hydrophilic substance has an affinity for water, which means it attracts water. Conversely, a hydrophobic substance is one that avoids water or does not have an affinity for water. For example, lipids are hydrophobic because they have relatively nonpolar bonds, while water is polar. Water is the most common molecule in living organisms, so it is important that we understand its interactions.
Hydrogen Bonding
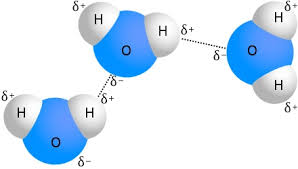
Image Credit: Chemicool
Hydrogen bonding is a type of chemical intermolecular bond that occurs when a hydrogen atom bonds to a highly electronegative atom, including oxygen, nitrogen, and fluorine. This creates a dipole moment in the molecule, where the hydrogen carries a partial positive charge, and the electronegative atom has a partial negative charge—the type of structure we observed that gives water its polarity, as water molecules bond together through hydrogen bonding. Hydrogen bonds are generally weaker than covalent bonds, but they are still relatively strong and play an important role in many chemical and biological processes.
In biology, hydrogen bonding is critical, as hydrogen bonding occurs in many different types of molecules, including water, DNA, and proteins. Hydrogen bonding is responsible for many molecular properties, such as the shape and function of proteins, the stability of many chemical compounds, and the formation of intermolecular interactions in crystalline solids. Hydrogen bonds between water molecules give water the properties of cohesion, adhesion, surface tension, specific heat, and evaporative cooling.
Properties of Water
1. Cohesion: the attraction of water molecules. Strong cohesive forces are present because they form hydrogen bonds with each other.
- Example: Cohesion due to hydrogen bonding contributes to the transport of water and nutrients against gravity in plants. Transpiration is the loss of water from a plant in the form of water vapor.
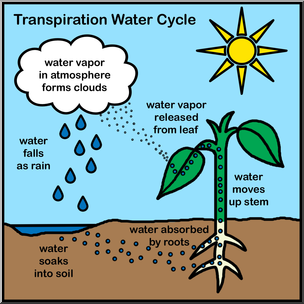
Image courtesy of ABCteach.
2. Adhesion: when one substance is attracted to another. Water adheres to other molecules and surfaces.
- Example: water on a glass surface, like a window or windshield.
3. Surface Tension: difficulty to break the surface of the water because of cohesive forces.
- Example: water strider insects rely on surface tension to stay afloat and walk on the surface of bodies of freshwater.
4. Specific Heat: the amount of heat energy it takes to raise or lower the temperature of one gram of a substance by one degree Celsius. Water has a high specific heat, so it can absorb or release a large amount of heat with only a slight change in its own temperature so large bodies of water take a while to evaporate.
5. Evaporative Cooling: water has a high heat of vaporization, so the water can absorb a lot of heat and leave the surface cooler.
- Example: excess body heat is used to convert beads of sweat/perspiration into vapor, which cools down the body
6. Dissociation of Water: hydrogen shifts from one water molecule to another.
-
When a molecule is increasing hydronium concentration by releasing hydrogen ions into solutions, it is an acid. (Ex. HCl ➡️ H+ + Cl-)
-
When a molecule is increasing hydroxide concentration by absorbing or accepting hydrogen ions, it is a base. (Ex: NaOH ➡️ Na+ + OH-) The counterbalance of hydronium and hydroxide results in water having a neutral pH of 7.0, meaning it is neither acidic nor basic.
-
pH scale range (0-14): acidic (< 7), neutral (7), alkaline/basic (> 7). Each level on the pH scale is a ten-fold change because the pH scale is logarithmic. Most biological fluids are in the pH range of 6-8. pH = −log [H+]
<< Hide Menu
1.1 Structure of Water and Hydrogen Bonding
4 min read•november 18, 2024
Danna Esther Gelfand
Jillian Holbrook
Danna Esther Gelfand
Jillian Holbrook
Skills you’ll gain in this topic:
- Understand how water’s polarity and hydrogen bonds create unique properties.
- Discuss how cohesion, adhesion, and surface tension support biological processes.
- Illustrate examples of water’s high specific heat and temperature stability effects.
- Analyze how water’s role as a solvent supports life at the molecular level.
- Connect water’s properties to its essential role in supporting organisms.
Water Molecules 💧
Water is a polar molecule, meaning the ends have opposite partial charges or unequal distribution of charge. The chemical formula of water H2O tells us that a water molecule is made up of two hydrogens and one oxygen. Water is polar because the hydrogens carry partial positive charges, and oxygen has a partial negative charge, which we can examine further when we discuss hydrogen bonding.
The simple phrase, "like attracts like," helps us to remember that polar substances are attracted to other polar substances, just as nonpolar substances are attracted to other nonpolar substances. Ever tried mixing oil and water? Oil molecules are nonpolar and have equally balanced charges rather than positive and negative poles like water. Because of this, oil and water are immiscible or do not combine. Polar and nonpolar do not attract because they are not alike.
In regard to water, there is specific terminology to describe attractive interactions. A hydrophilic substance has an affinity for water, which means it attracts water. Conversely, a hydrophobic substance is one that avoids water or does not have an affinity for water. For example, lipids are hydrophobic because they have relatively nonpolar bonds, while water is polar. Water is the most common molecule in living organisms, so it is important that we understand its interactions.
Hydrogen Bonding

Image Credit: Chemicool
Hydrogen bonding is a type of chemical intermolecular bond that occurs when a hydrogen atom bonds to a highly electronegative atom, including oxygen, nitrogen, and fluorine. This creates a dipole moment in the molecule, where the hydrogen carries a partial positive charge, and the electronegative atom has a partial negative charge—the type of structure we observed that gives water its polarity, as water molecules bond together through hydrogen bonding. Hydrogen bonds are generally weaker than covalent bonds, but they are still relatively strong and play an important role in many chemical and biological processes.
In biology, hydrogen bonding is critical, as hydrogen bonding occurs in many different types of molecules, including water, DNA, and proteins. Hydrogen bonding is responsible for many molecular properties, such as the shape and function of proteins, the stability of many chemical compounds, and the formation of intermolecular interactions in crystalline solids. Hydrogen bonds between water molecules give water the properties of cohesion, adhesion, surface tension, specific heat, and evaporative cooling.
Properties of Water
1. Cohesion: the attraction of water molecules. Strong cohesive forces are present because they form hydrogen bonds with each other.
- Example: Cohesion due to hydrogen bonding contributes to the transport of water and nutrients against gravity in plants. Transpiration is the loss of water from a plant in the form of water vapor.

Image courtesy of ABCteach.
2. Adhesion: when one substance is attracted to another. Water adheres to other molecules and surfaces.
- Example: water on a glass surface, like a window or windshield.
3. Surface Tension: difficulty to break the surface of the water because of cohesive forces.
- Example: water strider insects rely on surface tension to stay afloat and walk on the surface of bodies of freshwater.
4. Specific Heat: the amount of heat energy it takes to raise or lower the temperature of one gram of a substance by one degree Celsius. Water has a high specific heat, so it can absorb or release a large amount of heat with only a slight change in its own temperature so large bodies of water take a while to evaporate.
5. Evaporative Cooling: water has a high heat of vaporization, so the water can absorb a lot of heat and leave the surface cooler.
- Example: excess body heat is used to convert beads of sweat/perspiration into vapor, which cools down the body
6. Dissociation of Water: hydrogen shifts from one water molecule to another.
-
When a molecule is increasing hydronium concentration by releasing hydrogen ions into solutions, it is an acid. (Ex. HCl ➡️ H+ + Cl-)
-
When a molecule is increasing hydroxide concentration by absorbing or accepting hydrogen ions, it is a base. (Ex: NaOH ➡️ Na+ + OH-) The counterbalance of hydronium and hydroxide results in water having a neutral pH of 7.0, meaning it is neither acidic nor basic.
-
pH scale range (0-14): acidic (< 7), neutral (7), alkaline/basic (> 7). Each level on the pH scale is a ten-fold change because the pH scale is logarithmic. Most biological fluids are in the pH range of 6-8. pH = −log [H+]

© 2024 Fiveable Inc. All rights reserved.