3.3 Environmental Impacts on Enzyme Function
4 min read•november 18, 2024
Caroline Koffke
Caroline Koffke
Skills you’ll gain in this topic:
- Describe how temperature, pH, and substrate concentration affect enzyme function.
- Explain how denaturation alters enzyme structure and disrupts activity.
- Differentiate between competitive and noncompetitive inhibition of enzymes.
- Predict the effects of environmental changes on enzyme efficiency.
- Analyze the roles of cofactors and coenzymes in supporting enzyme function.
How Environment Affects Enzyme Activity
Changes in the environment will affect the speed in which an enzyme functions. Proteins that are denatured lose their function, but sometimes, this can be reversed, called renaturation. It's like curling your hair. Though you curl it, it'll probably come back to its straight form in a few days. This is like renaturing a protein. However, in most cases, denaturation is nonreversible. Think of boiling an egg. Once you boil it, you can't unboil it.
There are multiple ways to denature a protein, and this topic is a FRQ-likely topic, so keep it in mind!
Temperature
Temperature is able to speed up and slow down reactions. Usually, when the temperature is very cold, molecules are moving more slowly, and there are less opportunities for an enzyme and substrate to bump into one another. This slows the rate of the reaction and the effect of the enzyme.
Image courtesy of Giphy.
When temperatures increase, molecules move more quickly and bump into one another more frequently. Because of this, the rate of a reaction increases as temperature increases. That is until a specific threshold is hit. Once the temperature becomes too high, the enzyme begins to denature. This occurs when the bonds that hold the amino acids into their 3-dimensional shape begin to break. Once the enzyme loses its shape, it loses its active site and is unable to function. Remember, structure is important for function, so if the structure is significantly altered, the protein will essentially malfunction.
Therefore, enzymes have an optimal temperature range in which they function. For enzymes that work in the human body, they function best around our body temperature, 97-99 degrees Fahrenheit, or around 37 Celsius. When the temperature is too high or too low, the enzymes are not able to perform the life-sustaining reactions that they need to.
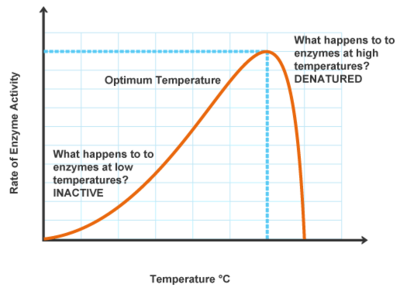
Image Courtesy of Pathwayz
pH
pH is a measurement referring to the number of hydrogen ions present in a solution. When there are a lot of hydrogen ions present, the solution has a low pH and is considered acidic. When there is a small number of hydrogen ions present, the solution has a high pH and is considered basic. Every enzyme has an optimal pH where it has its highest activity. A reduction or increase in pH outside of optimal pH leads to the enzyme's activity to slow down and possible denaturation of the enzyme or substrate (leading to a substrate being unable to bind to an active site).
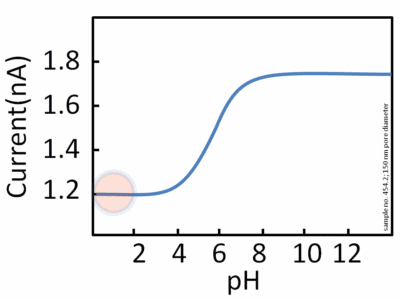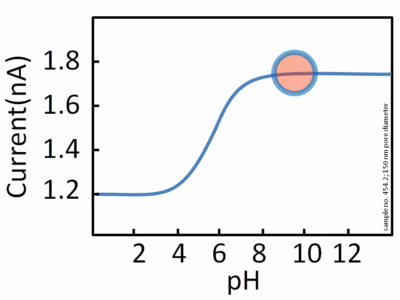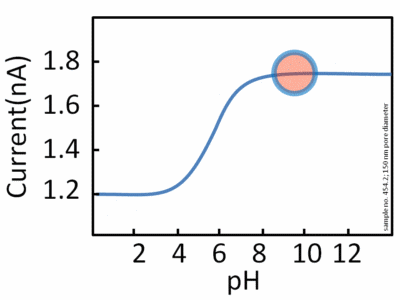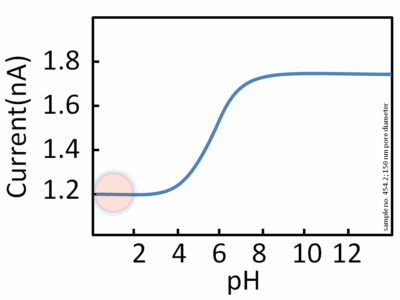
Image courtesy of WikiMedia Commons.
Most enzymes work at an optimal pH of 7, but it doesn't hold true for all enzymes. Some enzymes, such as pepsin work best at a pH of 2. This is because pepsin is a digestive enzyme that is found in the stomach, so it would make sense that this enzyme would work best at an acidic environment. Similarly, enzymes found in the lysosome function best at an acidic pH.
So why do enzymes get denatured at an incorrect pH? It's because hydrogen bonds can be altered by the pH level, thus altering the structure of the protein (and you guessed it) and thus its function.
Concentration
If the concentration of either is increased, the rate of the reaction should increase, as there is more opportunity for the two to meet. Ideally, both enzyme and substrate concentration would increase, as if only one increases, the other acts as a limiting reagent. This means that the rate of the reaction is limited by the amount of either enzyme or substrate available. An example of a limiting reagent is below.
The concentration of both enzymes and substrates can affect the overall rate of the reaction. A higher concentration of both enzyme and substrate makes it more likely that the two will bump into one another. If either is in low supply, there will be fewer molecules available to start a reaction.
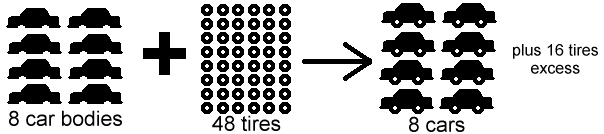
Image courtesy of Texas A&M University
As described above, there are a number of things that can alter the function of an enzyme, some of which can lead to denaturation. This process can either be temporary or permanent, depending on the magnitude of the damage.
The human body maintains strict ranges of temperature and pH in order to maintain the optimal functioning of enzymes. If the body enters a range that does not support enzyme functioning, such as very high temperature, the enzymes in the body may denature, and the person could die. Thankfully, your body has a number of checks and balances in place to ensure that optimal ranges are continually met.
Inhibitors
Inhibitors don't denature the protein, but they do alter the structure of the protein. Competitive inhibitors simply bind to the binding site that the substrate was intended to bind to. This doesn't alter the protein, it simply makes it less likely for the substrate to bind to its enzyme.
On the other hand, noncompetitive inhibitors alter the structure of the protein. Noncompetitive inhibitors do not necessarily bind to the binding site of the enzyme, instead, they bind somewhere else. But because they bind somewhere else, the original binding site gets altered, causing the intended substrate to not fit in.
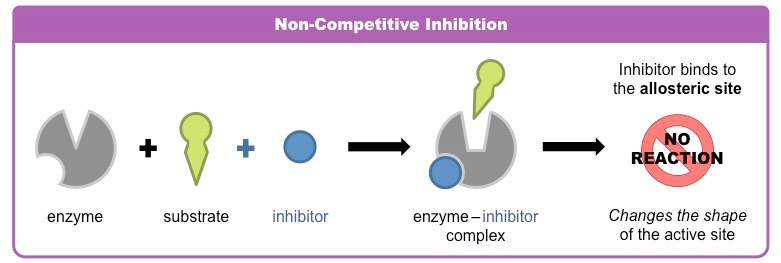
Image Courtesy of BioNinja
This can decrease the reaction rate. Both competitive and noncompetitive inhibitors can be reversible or irreversible, depending on the situation.
<< Hide Menu
3.3 Environmental Impacts on Enzyme Function
4 min read•november 18, 2024
Caroline Koffke
Caroline Koffke
Skills you’ll gain in this topic:
- Describe how temperature, pH, and substrate concentration affect enzyme function.
- Explain how denaturation alters enzyme structure and disrupts activity.
- Differentiate between competitive and noncompetitive inhibition of enzymes.
- Predict the effects of environmental changes on enzyme efficiency.
- Analyze the roles of cofactors and coenzymes in supporting enzyme function.
How Environment Affects Enzyme Activity
Changes in the environment will affect the speed in which an enzyme functions. Proteins that are denatured lose their function, but sometimes, this can be reversed, called renaturation. It's like curling your hair. Though you curl it, it'll probably come back to its straight form in a few days. This is like renaturing a protein. However, in most cases, denaturation is nonreversible. Think of boiling an egg. Once you boil it, you can't unboil it.
There are multiple ways to denature a protein, and this topic is a FRQ-likely topic, so keep it in mind!
Temperature
Temperature is able to speed up and slow down reactions. Usually, when the temperature is very cold, molecules are moving more slowly, and there are less opportunities for an enzyme and substrate to bump into one another. This slows the rate of the reaction and the effect of the enzyme.

Image courtesy of Giphy.
When temperatures increase, molecules move more quickly and bump into one another more frequently. Because of this, the rate of a reaction increases as temperature increases. That is until a specific threshold is hit. Once the temperature becomes too high, the enzyme begins to denature. This occurs when the bonds that hold the amino acids into their 3-dimensional shape begin to break. Once the enzyme loses its shape, it loses its active site and is unable to function. Remember, structure is important for function, so if the structure is significantly altered, the protein will essentially malfunction.
Therefore, enzymes have an optimal temperature range in which they function. For enzymes that work in the human body, they function best around our body temperature, 97-99 degrees Fahrenheit, or around 37 Celsius. When the temperature is too high or too low, the enzymes are not able to perform the life-sustaining reactions that they need to.

Image Courtesy of Pathwayz
pH
pH is a measurement referring to the number of hydrogen ions present in a solution. When there are a lot of hydrogen ions present, the solution has a low pH and is considered acidic. When there is a small number of hydrogen ions present, the solution has a high pH and is considered basic. Every enzyme has an optimal pH where it has its highest activity. A reduction or increase in pH outside of optimal pH leads to the enzyme's activity to slow down and possible denaturation of the enzyme or substrate (leading to a substrate being unable to bind to an active site).

Image courtesy of WikiMedia Commons.
Most enzymes work at an optimal pH of 7, but it doesn't hold true for all enzymes. Some enzymes, such as pepsin work best at a pH of 2. This is because pepsin is a digestive enzyme that is found in the stomach, so it would make sense that this enzyme would work best at an acidic environment. Similarly, enzymes found in the lysosome function best at an acidic pH.
So why do enzymes get denatured at an incorrect pH? It's because hydrogen bonds can be altered by the pH level, thus altering the structure of the protein (and you guessed it) and thus its function.
Concentration
If the concentration of either is increased, the rate of the reaction should increase, as there is more opportunity for the two to meet. Ideally, both enzyme and substrate concentration would increase, as if only one increases, the other acts as a limiting reagent. This means that the rate of the reaction is limited by the amount of either enzyme or substrate available. An example of a limiting reagent is below.
The concentration of both enzymes and substrates can affect the overall rate of the reaction. A higher concentration of both enzyme and substrate makes it more likely that the two will bump into one another. If either is in low supply, there will be fewer molecules available to start a reaction.
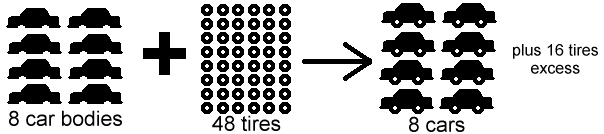
Image courtesy of Texas A&M University
As described above, there are a number of things that can alter the function of an enzyme, some of which can lead to denaturation. This process can either be temporary or permanent, depending on the magnitude of the damage.
The human body maintains strict ranges of temperature and pH in order to maintain the optimal functioning of enzymes. If the body enters a range that does not support enzyme functioning, such as very high temperature, the enzymes in the body may denature, and the person could die. Thankfully, your body has a number of checks and balances in place to ensure that optimal ranges are continually met.
Inhibitors
Inhibitors don't denature the protein, but they do alter the structure of the protein. Competitive inhibitors simply bind to the binding site that the substrate was intended to bind to. This doesn't alter the protein, it simply makes it less likely for the substrate to bind to its enzyme.
On the other hand, noncompetitive inhibitors alter the structure of the protein. Noncompetitive inhibitors do not necessarily bind to the binding site of the enzyme, instead, they bind somewhere else. But because they bind somewhere else, the original binding site gets altered, causing the intended substrate to not fit in.

Image Courtesy of BioNinja
This can decrease the reaction rate. Both competitive and noncompetitive inhibitors can be reversible or irreversible, depending on the situation.

© 2024 Fiveable Inc. All rights reserved.