Jed Quiaoit
Caroline Koffke
Jed Quiaoit
Caroline Koffke
Skills you’ll gain in this topic:
- Explain how enzymes facilitate chemical reactions and influence reaction rates.
- Describe how substrate concentration affects enzyme activity.
- Illustrate enzyme catalysis as a step-by-step process in metabolism.
- Identify factors impacting enzyme efficiency, including substrate availability.
- Relate enzyme activity to cellular energy management.
Remember… Constant Energy Input!
The highly complex organization of living systems refers to the many different processes that occur within cells and organisms, such as metabolism, growth, and reproduction. These processes involve the participation of many different biomolecules, including enzymes, which catalyze chemical reactions and are essential for maintaining the proper functioning of the cell or organism.
Enzyme catalysis plays an important role in the highly complex organization of living systems by allowing the cell to perform its functions more efficiently. For example, enzymes catalyze the breakdown of nutrients to generate energy, the synthesis of macromolecules such as DNA, RNA, and proteins, and the transfer of information between molecules. 🧬
This efficiency allows living systems to perform the wide range of chemical reactions necessary to sustain life while maintaining a relatively steady state, through constant input of energy and the exchange of macromolecules.
The constant input of energy is necessary to keep the enzymes functioning and also to power the chemical reactions that enzymes catalyze. Enzymes can be thought of as molecular machines that need energy to work properly, and their activity is usually coupled with energy-consuming reactions such as the transfer of electrons, proton, or ATP hydrolysis. 🤖
The exchange of macromolecules is another important aspect of the highly complex organization of living systems. Macromolecules, such as proteins and nucleic acids, are constantly being synthesized, degraded, and recycled. Enzymes play an important role in this process by catalyzing the synthesis and breakdown of these molecules.
The turnover of these molecules is crucial for maintaining the balance of the cell, the growth and the response to the environment!
Hold up! What is Catalysis?
Simply put, catalysis is the process by which a catalyst speeds up a chemical reaction. 🏃💨
Catalysts work by providing an alternative reaction pathway with a lower activation energy. In other words, catalysts can make a reaction proceed faster by providing an easier way for the reactants to transform into products. They can do this by:
- Changing the relative positions of atoms in the reactants, making it easier for them to form the products.
- Stabilizing the intermediate products or transition states, making the reaction proceed more smoothly.
- Providing an alternative and more favorable transition state for the reaction to proceed.
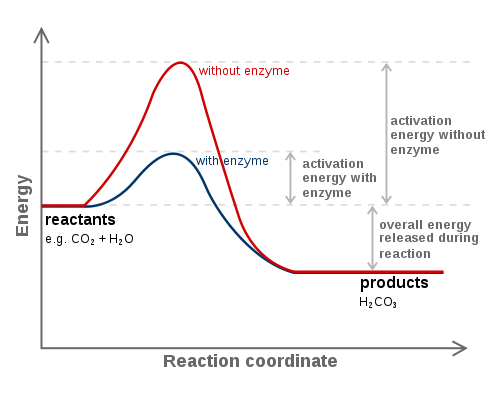
Source: Wikimedia Commons
How Does This Relate to Enzymes?
Enzymes are biomolecules that catalyze chemical reactions in cells. Just like any other catalyst in other disciplines, they do this by lowering the activation energy (the amount of energy required for a reaction to proceed) of the reaction they catalyze. This allows the reaction to occur more quickly and efficiently.
Enzymes are specific for the reactions they catalyze and the substrates (remember: substrates = reactants) they act on. They do this by having a unique three-dimensional structure that allows them to bind to the substrates in a specific way, a process called enzyme-substrate recognition. This binding forms an intermediate complex called the enzyme-substrate complex, which is more reactive than the substrate alone. 🧊
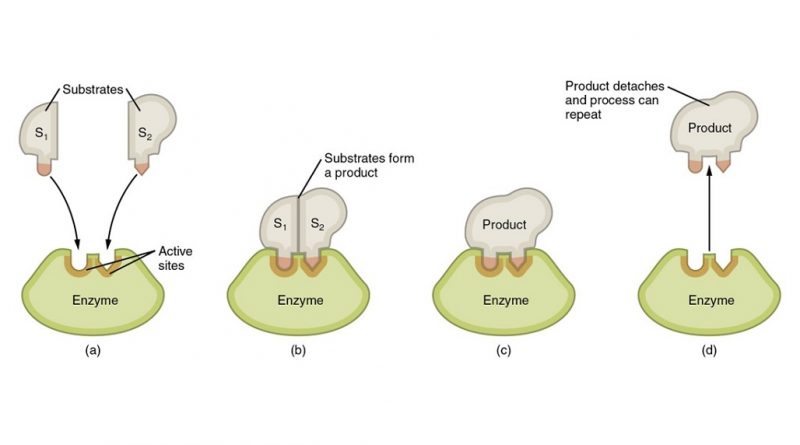
Source: Baylor College of Medicine
As the enzyme catalyzes the reaction, the substrate is converted into products, which then dissociate from the enzyme, leaving it free to bind to another substrate molecule. Enzymes can be reused several times in this way.
The rate of an enzyme-catalyzed reaction is affected by several factors, including the concentration of substrate, the presence of inhibitors or activators, and the temperature and pH of the environment.
An increase in substrate concentration can lead to an increase in the rate of an enzyme-catalyzed reaction, up to a certain point, after which the rate levels off. This is known as the saturation point.
Inhibitors are molecules that bind to enzymes and decrease the rate of reaction. They can do this by binding to the active site of the enzyme and preventing substrate binding, or by altering the shape of the enzyme so it cannot bind to the substrate.
Activators are molecules that bind to enzymes and increase the rate of reaction. They can do this by increasing the stability of the enzyme-substrate complex, making it more likely that the substrate will bind to the enzyme.
The temperature and pH of the environment also affect enzyme activity. Most enzymes have an optimal temperature and pH at which they function best. At temperatures or pHs outside this range, the enzyme may become denatured, or lose its shape, and can no longer function.
Competitive and Noncompetitive Inhibitors
A few substances act to inhibit or activate the processes of enzymes. While there are many environmental factors that can alter an enzyme’s ability to function, explained next, there are also certain molecules that can act to change the process as well. A chart of these molecules is shown below. 😡
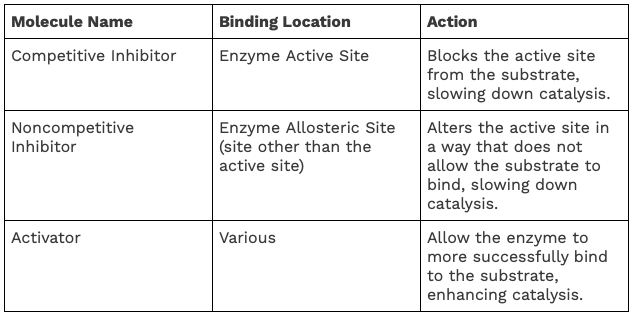
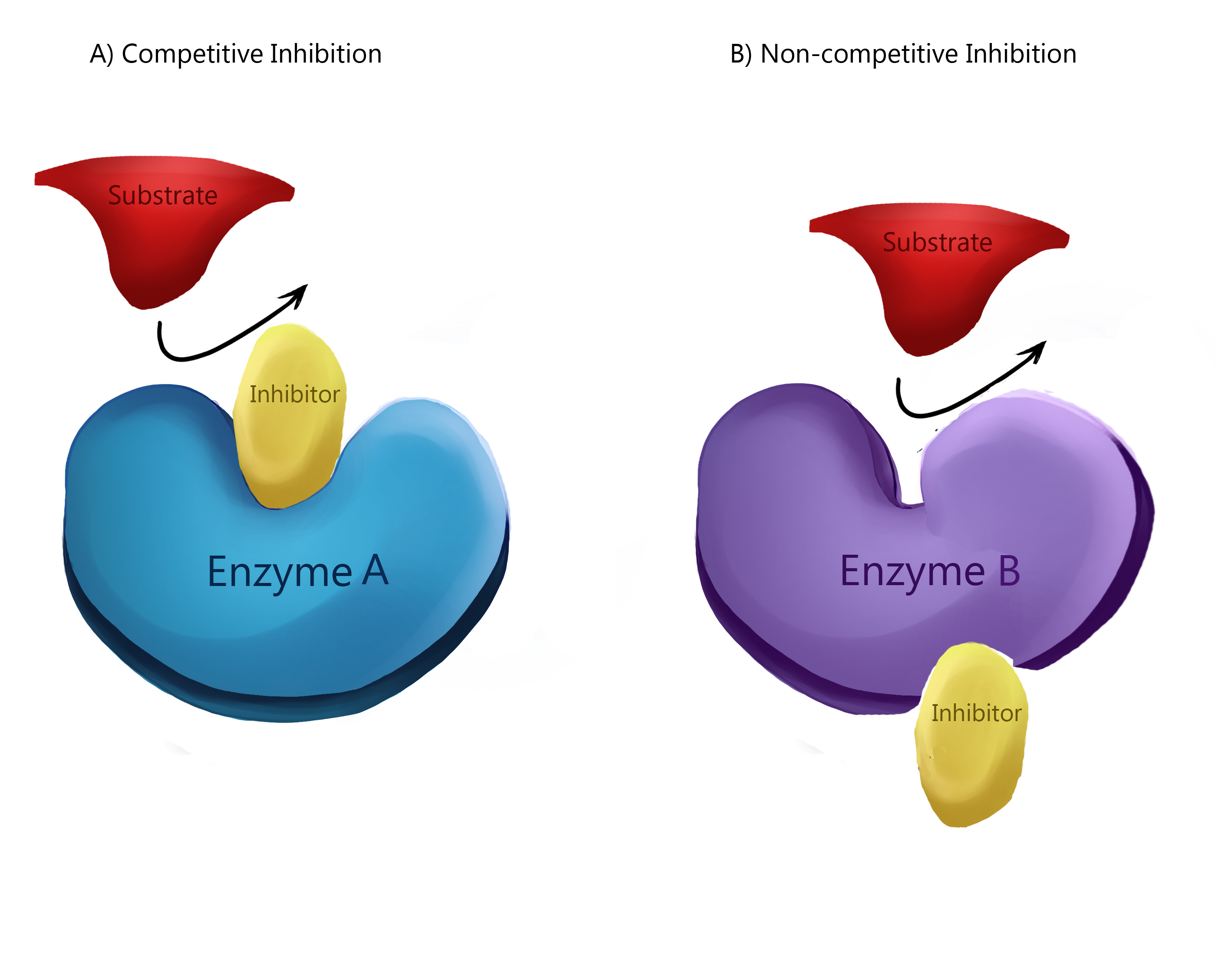
Source: WikiMedia Commons.
Test Your Knowledge
A scientist wants to study the effect of pH on the activity of an enzyme called "Protein X." He prepares a solution of the enzyme at a concentration of 0.1 mg/mL and adds it to a reaction mixture containing the substrate for the enzyme. He then measures the rate of the reaction at different pH levels. The table below shows his results.
| pH | Reaction Rate (mM/min) |
| 7.0 | 0.10 |
| 7.5 | 0.15 |
| 8.0 | 0.20 |
| 8.5 | 0.15 |
| 9.0 | 0.10 |
Based on these results, answer the following questions:
- At which pH level is the enzyme most active?
- How does the activity of the enzyme change as the pH deviates from its optimal range?
- How would the activity of the enzyme be affected if the pH deviated too far from the optimal range? What sort of changes to the enzyme might cause this to occur?
- In the living cells, the enzyme is surrounded by a variety of buffers that helps in maintaining the pH, what role do you think buffer play in this enzyme activity?
Answers
- The enzyme is most active at pH 8.0
- As the pH deviates from its optimal range, the activity of the enzyme decreases.
- The activity of the enzyme decreases significantly when the pH deviates too far from the optimal range because enzymes have a specific pH range in which they function optimally. If the pH deviates too far from this range, the enzyme may become denatured, or lose its shape, and can no longer function.
- Buffers plays an important role in maintaining the pH around enzymes, by removing hydrogen ions when the pH becomes too low and adding hydrogen ions when the pH becomes too high, buffers can help to keep the pH in the optimal range for enzyme activity.
<< Hide Menu
Jed Quiaoit
Caroline Koffke
Jed Quiaoit
Caroline Koffke
Skills you’ll gain in this topic:
- Explain how enzymes facilitate chemical reactions and influence reaction rates.
- Describe how substrate concentration affects enzyme activity.
- Illustrate enzyme catalysis as a step-by-step process in metabolism.
- Identify factors impacting enzyme efficiency, including substrate availability.
- Relate enzyme activity to cellular energy management.
Remember… Constant Energy Input!
The highly complex organization of living systems refers to the many different processes that occur within cells and organisms, such as metabolism, growth, and reproduction. These processes involve the participation of many different biomolecules, including enzymes, which catalyze chemical reactions and are essential for maintaining the proper functioning of the cell or organism.
Enzyme catalysis plays an important role in the highly complex organization of living systems by allowing the cell to perform its functions more efficiently. For example, enzymes catalyze the breakdown of nutrients to generate energy, the synthesis of macromolecules such as DNA, RNA, and proteins, and the transfer of information between molecules. 🧬
This efficiency allows living systems to perform the wide range of chemical reactions necessary to sustain life while maintaining a relatively steady state, through constant input of energy and the exchange of macromolecules.
The constant input of energy is necessary to keep the enzymes functioning and also to power the chemical reactions that enzymes catalyze. Enzymes can be thought of as molecular machines that need energy to work properly, and their activity is usually coupled with energy-consuming reactions such as the transfer of electrons, proton, or ATP hydrolysis. 🤖
The exchange of macromolecules is another important aspect of the highly complex organization of living systems. Macromolecules, such as proteins and nucleic acids, are constantly being synthesized, degraded, and recycled. Enzymes play an important role in this process by catalyzing the synthesis and breakdown of these molecules.
The turnover of these molecules is crucial for maintaining the balance of the cell, the growth and the response to the environment!
Hold up! What is Catalysis?
Simply put, catalysis is the process by which a catalyst speeds up a chemical reaction. 🏃💨
Catalysts work by providing an alternative reaction pathway with a lower activation energy. In other words, catalysts can make a reaction proceed faster by providing an easier way for the reactants to transform into products. They can do this by:
- Changing the relative positions of atoms in the reactants, making it easier for them to form the products.
- Stabilizing the intermediate products or transition states, making the reaction proceed more smoothly.
- Providing an alternative and more favorable transition state for the reaction to proceed.

Source: Wikimedia Commons
How Does This Relate to Enzymes?
Enzymes are biomolecules that catalyze chemical reactions in cells. Just like any other catalyst in other disciplines, they do this by lowering the activation energy (the amount of energy required for a reaction to proceed) of the reaction they catalyze. This allows the reaction to occur more quickly and efficiently.
Enzymes are specific for the reactions they catalyze and the substrates (remember: substrates = reactants) they act on. They do this by having a unique three-dimensional structure that allows them to bind to the substrates in a specific way, a process called enzyme-substrate recognition. This binding forms an intermediate complex called the enzyme-substrate complex, which is more reactive than the substrate alone. 🧊

Source: Baylor College of Medicine
As the enzyme catalyzes the reaction, the substrate is converted into products, which then dissociate from the enzyme, leaving it free to bind to another substrate molecule. Enzymes can be reused several times in this way.
The rate of an enzyme-catalyzed reaction is affected by several factors, including the concentration of substrate, the presence of inhibitors or activators, and the temperature and pH of the environment.
An increase in substrate concentration can lead to an increase in the rate of an enzyme-catalyzed reaction, up to a certain point, after which the rate levels off. This is known as the saturation point.
Inhibitors are molecules that bind to enzymes and decrease the rate of reaction. They can do this by binding to the active site of the enzyme and preventing substrate binding, or by altering the shape of the enzyme so it cannot bind to the substrate.
Activators are molecules that bind to enzymes and increase the rate of reaction. They can do this by increasing the stability of the enzyme-substrate complex, making it more likely that the substrate will bind to the enzyme.
The temperature and pH of the environment also affect enzyme activity. Most enzymes have an optimal temperature and pH at which they function best. At temperatures or pHs outside this range, the enzyme may become denatured, or lose its shape, and can no longer function.
Competitive and Noncompetitive Inhibitors
A few substances act to inhibit or activate the processes of enzymes. While there are many environmental factors that can alter an enzyme’s ability to function, explained next, there are also certain molecules that can act to change the process as well. A chart of these molecules is shown below. 😡


Source: WikiMedia Commons.
Test Your Knowledge
A scientist wants to study the effect of pH on the activity of an enzyme called "Protein X." He prepares a solution of the enzyme at a concentration of 0.1 mg/mL and adds it to a reaction mixture containing the substrate for the enzyme. He then measures the rate of the reaction at different pH levels. The table below shows his results.
| pH | Reaction Rate (mM/min) |
| 7.0 | 0.10 |
| 7.5 | 0.15 |
| 8.0 | 0.20 |
| 8.5 | 0.15 |
| 9.0 | 0.10 |
Based on these results, answer the following questions:
- At which pH level is the enzyme most active?
- How does the activity of the enzyme change as the pH deviates from its optimal range?
- How would the activity of the enzyme be affected if the pH deviated too far from the optimal range? What sort of changes to the enzyme might cause this to occur?
- In the living cells, the enzyme is surrounded by a variety of buffers that helps in maintaining the pH, what role do you think buffer play in this enzyme activity?
Answers
- The enzyme is most active at pH 8.0
- As the pH deviates from its optimal range, the activity of the enzyme decreases.
- The activity of the enzyme decreases significantly when the pH deviates too far from the optimal range because enzymes have a specific pH range in which they function optimally. If the pH deviates too far from this range, the enzyme may become denatured, or lose its shape, and can no longer function.
- Buffers plays an important role in maintaining the pH around enzymes, by removing hydrogen ions when the pH becomes too low and adding hydrogen ions when the pH becomes too high, buffers can help to keep the pH in the optimal range for enzyme activity.

© 2024 Fiveable Inc. All rights reserved.