Jed Quiaoit
Caroline Koffke
Jed Quiaoit
Caroline Koffke
Skills you’ll gain in this topic:
- Describe the structure of enzymes and their active sites.
- Explain how enzymes lower activation energy in biochemical reactions.
- Illustrate how enzyme specificity impacts substrate interactions.
- Predict how changes in enzyme structure affect activity.
- Relate enzyme structure to its role in cellular processes.
Living Organisms and Energy
One of the ways in which living systems maintain their highly complex organization is through the constant input of energy. This energy is typically obtained through metabolic processes, such as respiration or photosynthesis, which involve the conversion of nutrients into usable energy. In addition to providing the energy needed to fuel cellular processes, this constant input of energy also helps to maintain the structural integrity of the cell. For these processes to occur, enzymes are required.
Enzymes are a crucial component of the highly complex organization of living systems. These specialized proteins act as catalysts, speeding up chemical reactions within cells and enabling them to carry out the many functions necessary for life. Enzymes are involved in a wide range of cellular processes, including metabolism, cell division, and gene expression, and they are essential for the proper functioning of cells. ⚡
Source: Wikimedia Commons
Shape of Enzymes
It is important to understand the structure of enzymes and how it relates to their function as enzymes are the workhorses of the cell, catalyzing the chemical reactions necessary for metabolism, growth, and reproduction. The structure of enzymes plays a critical role in their ability to carry out these functions. ⚪
Enzymes are composed of one or more polypeptide chains, which are long chains of amino acids. The specific sequence of amino acids in an enzyme determines its primary structure. The primary structure is the linear sequence of amino acids in a polypeptide or protein. However, primary structure alone cannot account for the complexity and diversity of enzyme function, this is where the higher levels of structure come into play. 🐜
The specific arrangement of the amino acid residues in space determines the enzyme's three-dimensional structure, which is crucial for its function. The three-dimensional structure of enzymes can be divided into several levels:
- Secondary structure, which refers to the local patterning of the amino acids, such as the formation of alpha-helices or beta-sheets.
- Tertiary structure, which refers to the overall three-dimensional conformation of the enzyme, including the location of the active site and other functional groups.
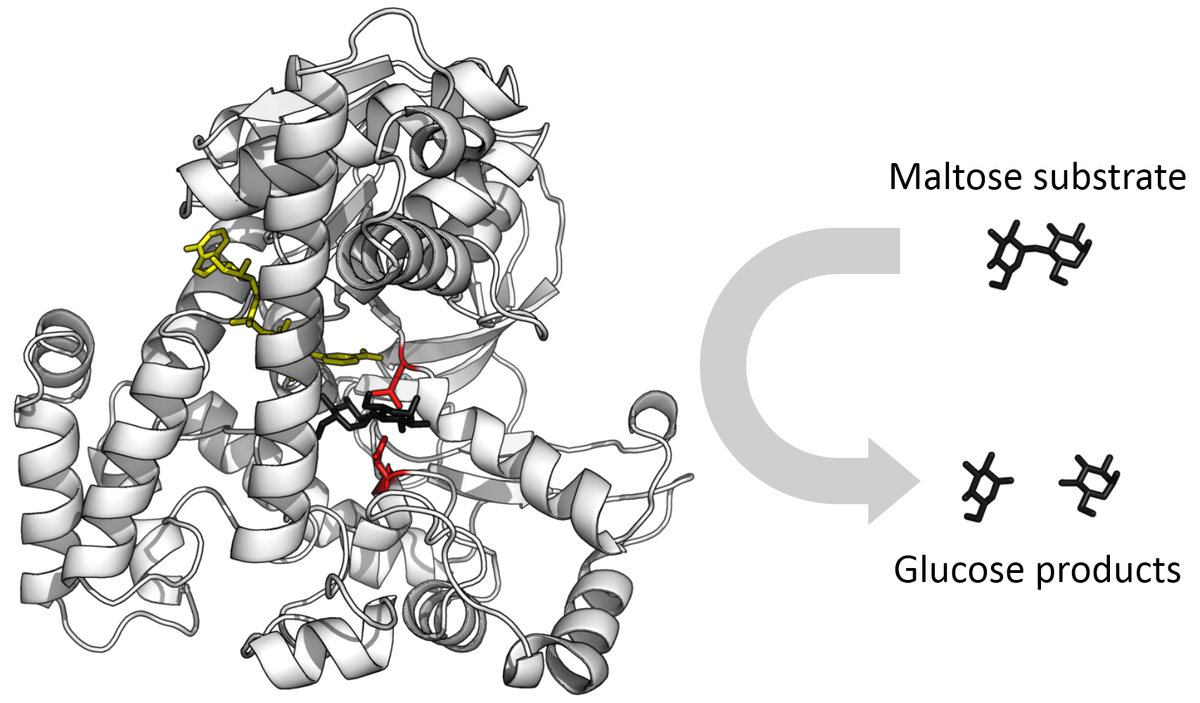
- Quaternary structure, which refers to the spatial relationship between the subunits in multimeric enzymes (Multimeric refers to a complex of multiple identical or non-identical subunits). Enzymes function by binding to substrate molecules and catalyzing specific chemical reactions. The active site of an enzyme is the region of the enzyme where the substrate binds and the reaction takes place. The three-dimensional structure of the enzyme, including the active site, must be complementary to the substrate in order for the enzyme to bind to it and catalyze the reaction.
Source: WikiMedia Commons.
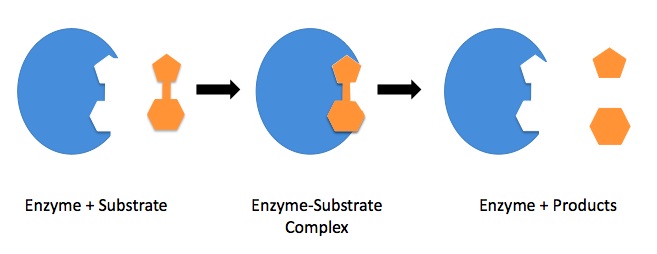
Furthermore, enzymes are dynamic molecules that can change shape upon binding to substrate or other molecules, this is known as the induced fit mechanism. This conformational change can enhance the specificity and efficiency of the enzyme-substrate interaction, thus increasing the rate of the chemical reaction.
Source: WikiMedia Commons.
Active Site
Enzymes have a specific region called the active site, which is where the substrate(s) bind and the chemical reaction takes place. The active site is usually a depression or cleft on the surface of the enzyme and is often lined with specific amino acids that interact with the substrate. 🧑🤝🧑
Enzymes are highly specific and only catalyze specific reactions. This specificity is due to the specific shape of the active site, which only fits the substrate for which it is intended.
In order for an enzyme-mediated chemical reaction to occur, the substrate must first bind to the active site of the enzyme. The active site is a specific region on the surface of the enzyme that is designed to interact with the substrate.
The shape and charge of the substrate must be compatible with the active site of the enzyme in order for the substrate to bind effectively. This is because the active site is specifically shaped to fit the substrate, and the amino acids that make up the active site often have specific charges that interact with the substrate.
If the shape or charge of the substrate is not compatible with the active site of the enzyme, the substrate will not bind effectively, and the chemical reaction will not occur.
Likewise, enzymes can be regulated to control the rate of a reaction. This can be done by several mechanisms, including allosteric regulation, where a molecule binds to a specific site on the enzyme (called an allosteric site) and changes the shape of the active site, causing the enzyme to either become more or less active. Enzymes can also be regulated by the concentration of substrate or the presence of an inhibitor. 👎
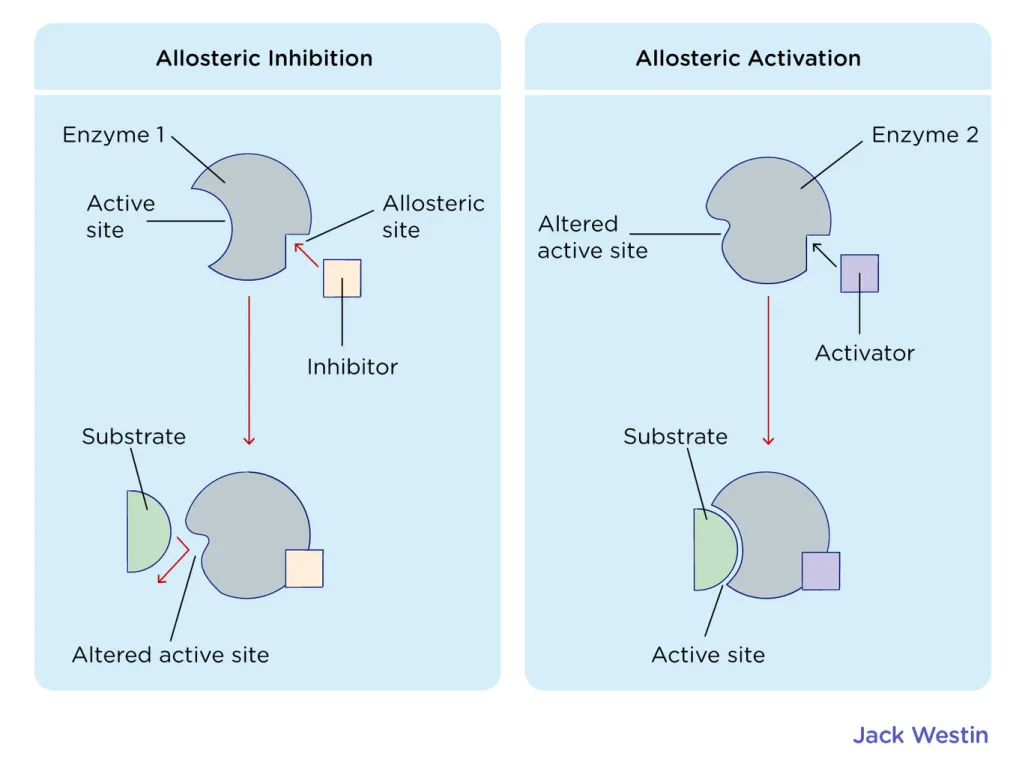
Source: Jack Westin
Induced Fit
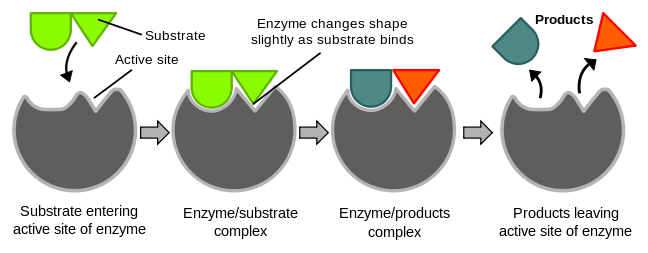
Induced fit is a mechanism (model) of enzyme catalysis in which the enzyme changes its conformation or shape upon binding to the substrate, resulting in a tighter and more specific binding of the enzyme and substrate, and ultimately, a more efficient catalytic reaction. 🧤
In induced fit, the enzyme's active site is not a rigid, pre-formed structure that exactly matches the substrate's shape (think of a glove snugly fitting into your hand), but rather a flexible structure that adjusts its conformation upon binding to the substrate. As the substrate enters the active site, the enzyme's amino acid residues in the active site move slightly to adjust their positions, resulting in a tighter fit between the enzyme and substrate. This tighter fit allows for more efficient formation of the transition state, which is the high-energy intermediate between the substrate and the products, and hence increases the rate of the reaction.
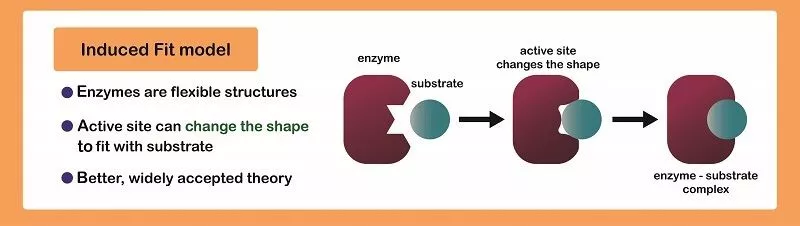
Source: Biology Dictionary
This mechanism of enzyme catalysis can also play a role in substrate specificity. When the enzyme's active site is flexible, it can adjust its conformation to fit a variety of different substrates. However, the adjustments it makes to bind different substrates may be different, and only the substrate that fits the active site the best will be catalyzed with the highest efficiency.
In addition, induced fit can also contribute to the regulation of enzyme activity by controlling the rate of substrate binding and product release. Some enzymes can tightly bind the substrate only in certain conditions such as the presence of a cofactor or a specific environment, in these cases the induced fit mechanism is more relevant for the regulation of the enzyme activity than for substrate specificity.
<< Hide Menu
Jed Quiaoit
Caroline Koffke
Jed Quiaoit
Caroline Koffke
Skills you’ll gain in this topic:
- Describe the structure of enzymes and their active sites.
- Explain how enzymes lower activation energy in biochemical reactions.
- Illustrate how enzyme specificity impacts substrate interactions.
- Predict how changes in enzyme structure affect activity.
- Relate enzyme structure to its role in cellular processes.
Living Organisms and Energy
One of the ways in which living systems maintain their highly complex organization is through the constant input of energy. This energy is typically obtained through metabolic processes, such as respiration or photosynthesis, which involve the conversion of nutrients into usable energy. In addition to providing the energy needed to fuel cellular processes, this constant input of energy also helps to maintain the structural integrity of the cell. For these processes to occur, enzymes are required.
Enzymes are a crucial component of the highly complex organization of living systems. These specialized proteins act as catalysts, speeding up chemical reactions within cells and enabling them to carry out the many functions necessary for life. Enzymes are involved in a wide range of cellular processes, including metabolism, cell division, and gene expression, and they are essential for the proper functioning of cells. ⚡

Source: Wikimedia Commons
Shape of Enzymes
It is important to understand the structure of enzymes and how it relates to their function as enzymes are the workhorses of the cell, catalyzing the chemical reactions necessary for metabolism, growth, and reproduction. The structure of enzymes plays a critical role in their ability to carry out these functions. ⚪
Enzymes are composed of one or more polypeptide chains, which are long chains of amino acids. The specific sequence of amino acids in an enzyme determines its primary structure. The primary structure is the linear sequence of amino acids in a polypeptide or protein. However, primary structure alone cannot account for the complexity and diversity of enzyme function, this is where the higher levels of structure come into play. 🐜
The specific arrangement of the amino acid residues in space determines the enzyme's three-dimensional structure, which is crucial for its function. The three-dimensional structure of enzymes can be divided into several levels:
- Secondary structure, which refers to the local patterning of the amino acids, such as the formation of alpha-helices or beta-sheets.
- Tertiary structure, which refers to the overall three-dimensional conformation of the enzyme, including the location of the active site and other functional groups.
- Quaternary structure, which refers to the spatial relationship between the subunits in multimeric enzymes (Multimeric refers to a complex of multiple identical or non-identical subunits). Enzymes function by binding to substrate molecules and catalyzing specific chemical reactions. The active site of an enzyme is the region of the enzyme where the substrate binds and the reaction takes place. The three-dimensional structure of the enzyme, including the active site, must be complementary to the substrate in order for the enzyme to bind to it and catalyze the reaction.

Source: WikiMedia Commons.
Furthermore, enzymes are dynamic molecules that can change shape upon binding to substrate or other molecules, this is known as the induced fit mechanism. This conformational change can enhance the specificity and efficiency of the enzyme-substrate interaction, thus increasing the rate of the chemical reaction.

Source: WikiMedia Commons.
Active Site
Enzymes have a specific region called the active site, which is where the substrate(s) bind and the chemical reaction takes place. The active site is usually a depression or cleft on the surface of the enzyme and is often lined with specific amino acids that interact with the substrate. 🧑🤝🧑
Enzymes are highly specific and only catalyze specific reactions. This specificity is due to the specific shape of the active site, which only fits the substrate for which it is intended.
In order for an enzyme-mediated chemical reaction to occur, the substrate must first bind to the active site of the enzyme. The active site is a specific region on the surface of the enzyme that is designed to interact with the substrate.
The shape and charge of the substrate must be compatible with the active site of the enzyme in order for the substrate to bind effectively. This is because the active site is specifically shaped to fit the substrate, and the amino acids that make up the active site often have specific charges that interact with the substrate.
If the shape or charge of the substrate is not compatible with the active site of the enzyme, the substrate will not bind effectively, and the chemical reaction will not occur.
Likewise, enzymes can be regulated to control the rate of a reaction. This can be done by several mechanisms, including allosteric regulation, where a molecule binds to a specific site on the enzyme (called an allosteric site) and changes the shape of the active site, causing the enzyme to either become more or less active. Enzymes can also be regulated by the concentration of substrate or the presence of an inhibitor. 👎

Source: Jack Westin
Induced Fit
Induced fit is a mechanism (model) of enzyme catalysis in which the enzyme changes its conformation or shape upon binding to the substrate, resulting in a tighter and more specific binding of the enzyme and substrate, and ultimately, a more efficient catalytic reaction. 🧤
In induced fit, the enzyme's active site is not a rigid, pre-formed structure that exactly matches the substrate's shape (think of a glove snugly fitting into your hand), but rather a flexible structure that adjusts its conformation upon binding to the substrate. As the substrate enters the active site, the enzyme's amino acid residues in the active site move slightly to adjust their positions, resulting in a tighter fit between the enzyme and substrate. This tighter fit allows for more efficient formation of the transition state, which is the high-energy intermediate between the substrate and the products, and hence increases the rate of the reaction.

Source: Biology Dictionary
This mechanism of enzyme catalysis can also play a role in substrate specificity. When the enzyme's active site is flexible, it can adjust its conformation to fit a variety of different substrates. However, the adjustments it makes to bind different substrates may be different, and only the substrate that fits the active site the best will be catalyzed with the highest efficiency.
In addition, induced fit can also contribute to the regulation of enzyme activity by controlling the rate of substrate binding and product release. Some enzymes can tightly bind the substrate only in certain conditions such as the presence of a cofactor or a specific environment, in these cases the induced fit mechanism is more relevant for the regulation of the enzyme activity than for substrate specificity.

© 2024 Fiveable Inc. All rights reserved.