Jed Quiaoit
Caroline Koffke
Jed Quiaoit
Caroline Koffke
Cells Require Energy?!
Cellular energetics is the study of the production, distribution, and use of energy in cells. In the context of AP Biology, cellular energetics typically refers to the production of ATP (adenosine triphosphate) through cellular respiration and photosynthesis.
In cellular respiration, glucose is broken down through a series of reactions to produce ATP, which is then used by cells to power various cellular processes. This process occurs in the mitochondria of eukaryotic cells and in the cytoplasm of prokaryotic cells. 🦠
Photosynthesis is the process by which plants, algae, and some bacteria convert energy from sunlight into chemical energy stored in glucose or other organic molecules. This process occurs in the chloroplasts of plant cells and in the photosynthetic pigments of algae and bacteria.
Both cellular respiration and photosynthesis are important for the production of ATP, which is the primary source of energy for cells. Understanding how cells produce and use energy is a fundamental concept in biology and is essential for understanding many aspects of cellular biology and metabolism.
3.1 - 3.3 Enzymes
Enzymes are proteins that play a vital role in the chemical reactions that occur within cells. They help to speed up the rate of these reactions, which are essential for maintaining life. 🏃
One key feature of enzymes is the active site, which is a specific region of the enzyme that interacts with substrate molecules (reactants). The shape and charge of the substrate must be compatible with the active site in order for an enzyme-mediated chemical reaction to occur.
Enzymes help to regulate biological processes by facilitating chemical reactions and lowering the activation energy required for these reactions to occur. However, changes to the molecular structure of a component in an enzymatic system may result in a change of the function or efficiency of the system.
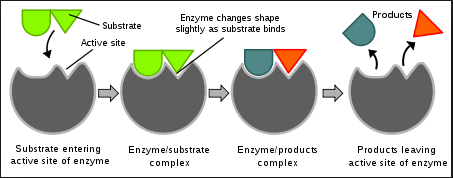
Source: Quizlet
For example, denaturation of an enzyme occurs when the protein structure is disrupted, eliminating the ability to catalyze reactions. This can be caused by environmental factors such as temperature and pH, which may be outside the optimal range for a given enzyme. In some cases, enzyme denaturation is reversible, allowing the enzyme to regain activity.
One way that environmental pH can impact enzyme activity is through the disruption of hydrogen bonds. These bonds are important for maintaining the three-dimensional structure of the enzyme.
The relative concentrations of substrates and products also play a role in determining the efficiency of an enzymatic reaction. If the concentration of the substrate is too low, the enzyme may not have enough molecules to react with, and the reaction will proceed more slowly. On the other hand, if the concentration of the product is too high, it can inhibit the enzyme's activity by occupying the active site, preventing the substrate from binding.
Higher temperatures can increase the speed of movement of molecules in a solution, which can lead to an increase in the rate of an enzymatic reaction. This is because increased movement increases the frequency of collisions between enzymes and substrates, making the reaction more likely to occur. 🥵
Competitive inhibitors are molecules that can bind to the active site of an enzyme, blocking the substrate from binding. These inhibitors can bind reversibly, meaning they can dissociate from the enzyme, or irreversibly, meaning they bind permanently. Noncompetitive inhibitors, on the other hand, bind to allosteric sites on the enzyme, which are not the active site. These inhibitors change the activity of the enzyme by causing it to change shape, and as a result, the substrate can no longer bind to the active site.
3.4 Energy
All living systems require a constant input of energy in order to maintain their highly ordered state and to power cellular processes. This is because life requires a highly ordered system, and the second law of thermodynamics dictates that in any closed system, entropy, or the measure of disorder, will always increase over time.
Cellular processes that release energy, such as metabolism, are often coupled with processes that require energy, such as the synthesis of molecules or the pumping of ions across a membrane. This allows the organism to harness the energy released by one process to power another.
If the input of energy into a system drops below the energy loss, or if the flow of energy is disrupted, the system will become disordered and will eventually reach a state of equilibrium, resulting in death.
Source: ThoughtCo
In biological systems, energy-related pathways are often sequential, with the product of one reaction serving as the reactant for the next step in the pathway. This allows for a more controlled and efficient transfer of energy, as the energy released by one reaction can be used to drive the next. This can lead to the production of more energy than if the reactions were to occur independently. This is the basis of the electron transport chain (ETC), as we will discuss later!
3.5 Photosynthesis
Organisms capture and store energy from a variety of sources for use in biological processes. One of the most important ways that organisms capture energy is through photosynthesis, which involves the capture of energy from the sun and the production of sugars. 🍭
Photosynthesis first evolved in prokaryotic organisms, which are single-celled organisms that lack a nucleus and other membrane-bound organelles. There is scientific evidence to support the claim that prokaryotic (cyanobacterial) photosynthesis was responsible for the production of an oxygenated atmosphere on Earth. These prokaryotic photosynthetic pathways were the foundation for the development of eukaryotic photosynthesis, which occurs in organisms with a nucleus and other membrane-bound organelles.
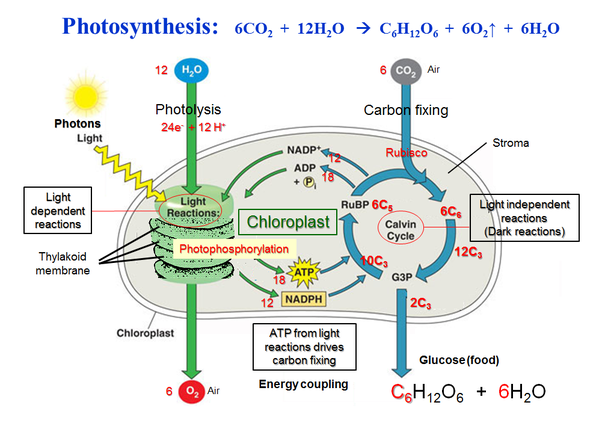
The light-dependent reactions of photosynthesis in eukaryotes involve a series of coordinated reaction pathways that capture energy present in light and convert it into ATP and NADPH, which are used to power the production of organic molecules.
Source: Quizlet
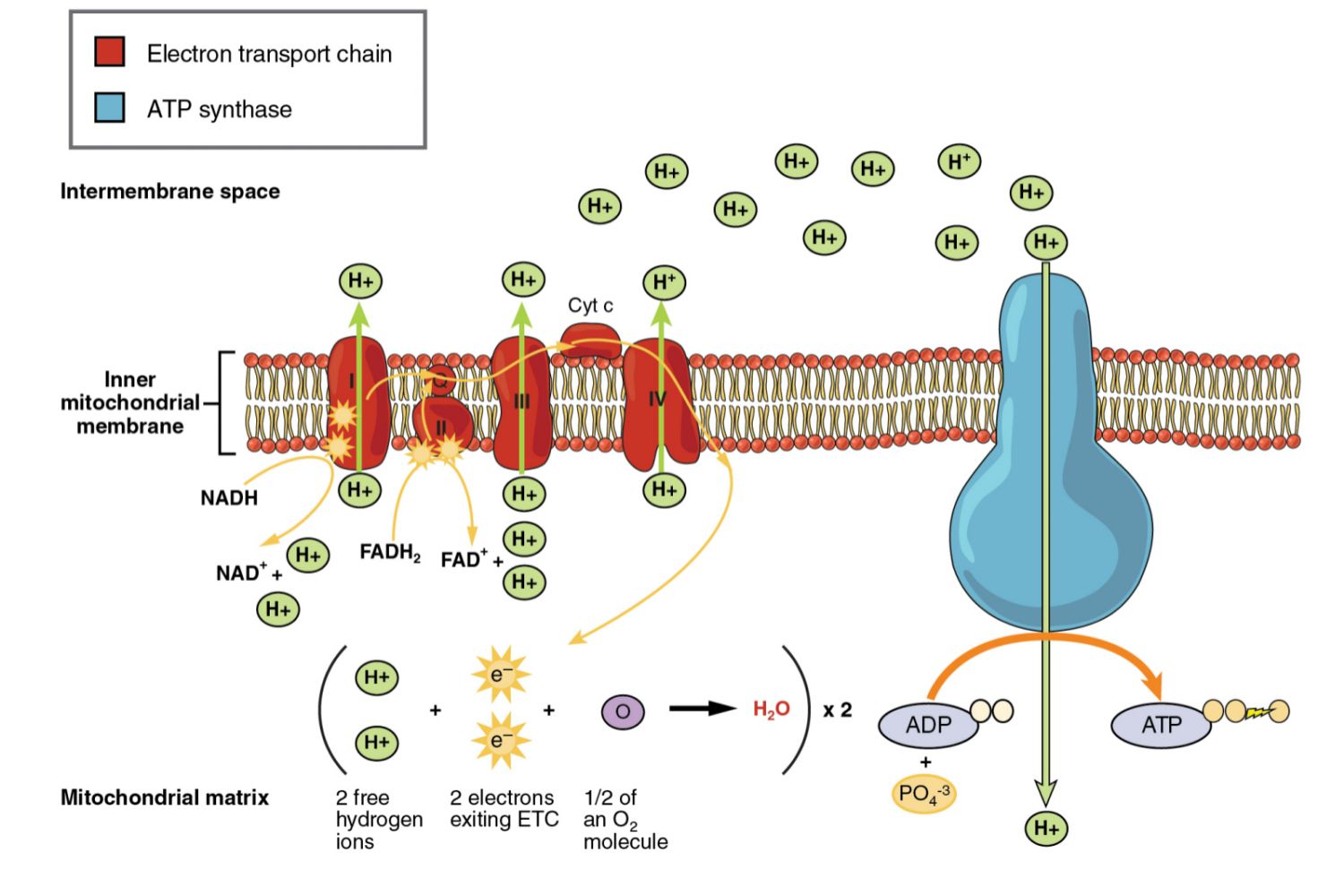
During photosynthesis, chlorophylls absorb energy from light, boosting electrons to a higher energy level in photosystems I and II. These photosystems are embedded in the internal membranes of chloroplasts, which are the organelles responsible for photosynthesis in plants and algae. They are connected by the transfer of higher energy electrons through an electron transport chain (ETC). The ETC generates a proton gradient that is used to produce ATP, which is the primary source of energy for cellular processes in most organisms. ⚡
When electrons are transferred between molecules in a sequence of reactions as they pass through the electron transport chain (ETC), an electrochemical gradient of protons (hydrogen ions) is established across the internal membrane of the cell.
The proton gradient is used to synthesize ATP from ADP and inorganic phosphate via ATP synthase, an enzyme that is embedded in the membrane. ATP synthase uses the energy from the proton gradient to power the synthesis of ATP, which is the primary source of energy for most cellular processes. ➕
The energy captured in the light reactions of photosynthesis and transferred to ATP and NADPH powers the production of carbohydrates from carbon dioxide in the Calvin cycle, which occurs in the stroma of the chloroplast. The Calvin cycle is a series of enzyme-catalyzed reactions that use the energy from ATP and NADPH to fix carbon dioxide into organic molecules, such as glucose.
3.6 Cellular Respiration and Fermentation
Fermentation and cellular respiration are two processes that use energy from biological macromolecules to produce ATP. These processes are characteristic of all forms of life and play a vital role in the production of energy in cells. Fermentation is an anaerobic process that occurs in the absence of oxygen and results in the production of ATP through the conversion of organic molecules into simpler products, such as lactate or ethanol. 🍺
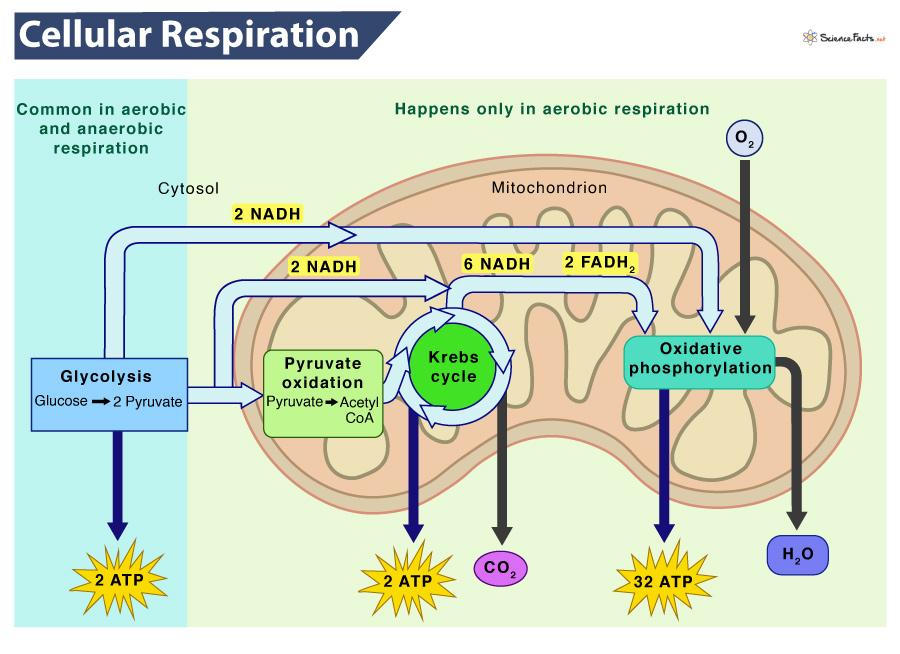
Cellular respiration is an aerobic process that occurs in the presence of oxygen and involves a series of coordinated enzyme-catalyzed reactions that capture energy from biological macromolecules, such as glucose. In eukaryotes, cellular respiration occurs in the mitochondria, which are the organelles responsible for energy production in the cell. The energy from cellular respiration is used to synthesize ATP.
In cellular respiration, electrons delivered by NADH and FADH are passed to a series of electron acceptors as they move toward the terminal electron acceptor, oxygen. In photosynthesis, the terminal electron acceptor is NADP+. Aerobic prokaryotes use oxygen as a terminal electron acceptor, while anaerobic prokaryotes use other molecules.
The flow of protons back through membrane-bound ATP synthase drives the formation of ATP from ADP and inorganic phosphate. This process is known as oxidative phosphorylation in cellular respiration and photophosphorylation in photosynthesis. In cellular respiration, decoupling oxidative phosphorylation from electron transport generates heat. This heat can be used by endothermic organisms to regulate body temperature. 🌡
Source: Science Facts
Glycolysis is a biochemical pathway that releases energy from glucose to form ATP from ADP and inorganic phosphate, NADH from NAD+, and pyruvate. This process occurs in the cytosol of cells and is the first step in the breakdown of glucose for energy.
Pyruvate, the end product of glycolysis, is then transported from the cytosol to the mitochondrion, where it undergoes further oxidation. In the mitochondrion, the Krebs cycle, also known as the citric acid cycle, occurs.
The Krebs cycle is a series of enzyme-catalyzed reactions that release energy from organic intermediates, produce carbon dioxide as a waste product, synthesize ATP from ADP and inorganic phosphate, and transfer electrons to the coenzymes NADH and FADH2.
The electrons extracted in glycolysis and the Krebs cycle reactions are then transferred by NADH and FADH2 to the electron transport chain (ETC) in the inner mitochondrial membrane. When electrons are transferred between molecules in a sequence of reactions as they pass through the ETC, an electrochemical gradient of protons (hydrogen ions) across the inner mitochondrial membrane is established. 😁
3.7 Fitness
Variation at the molecular level allows organisms to respond to a variety of environmental stimuli and can provide a greater ability to survive and/or reproduce in different environments. This variation can occur in the number and types of molecules within cells, and it is an important factor in the adaptability of organisms to their surroundings. 💪
Major Formulas
- Cellular Respiration: C6H12O6 + O2 → H2O + CO2
- Photosynthesis: H2O + CO2 → C6H12O6 + O2
- Other key components
- Electron carriers (NADH, FADH2, NADPH)
- Electron carriers are responsible for carrying electrons in the form of a hydrogen ion. They drop these electrons either at the electron transport chain in order to make ATP or in the Calvin Cycle to contribute to bond formation.
- ATP Synthase
- ATP synthase is the enzyme responsible for synthesizing ATP. Remember that enzymes end in -ase. This enzyme harnesses the power of chemiosmosis in order to phosphorylate ADP into ATP.
- Electron carriers (NADH, FADH2, NADPH)
<< Hide Menu
Jed Quiaoit
Caroline Koffke
Jed Quiaoit
Caroline Koffke
Cells Require Energy?!
Cellular energetics is the study of the production, distribution, and use of energy in cells. In the context of AP Biology, cellular energetics typically refers to the production of ATP (adenosine triphosphate) through cellular respiration and photosynthesis.
In cellular respiration, glucose is broken down through a series of reactions to produce ATP, which is then used by cells to power various cellular processes. This process occurs in the mitochondria of eukaryotic cells and in the cytoplasm of prokaryotic cells. 🦠
Photosynthesis is the process by which plants, algae, and some bacteria convert energy from sunlight into chemical energy stored in glucose or other organic molecules. This process occurs in the chloroplasts of plant cells and in the photosynthetic pigments of algae and bacteria.
Both cellular respiration and photosynthesis are important for the production of ATP, which is the primary source of energy for cells. Understanding how cells produce and use energy is a fundamental concept in biology and is essential for understanding many aspects of cellular biology and metabolism.
3.1 - 3.3 Enzymes
Enzymes are proteins that play a vital role in the chemical reactions that occur within cells. They help to speed up the rate of these reactions, which are essential for maintaining life. 🏃
One key feature of enzymes is the active site, which is a specific region of the enzyme that interacts with substrate molecules (reactants). The shape and charge of the substrate must be compatible with the active site in order for an enzyme-mediated chemical reaction to occur.
Enzymes help to regulate biological processes by facilitating chemical reactions and lowering the activation energy required for these reactions to occur. However, changes to the molecular structure of a component in an enzymatic system may result in a change of the function or efficiency of the system.

Source: Quizlet
For example, denaturation of an enzyme occurs when the protein structure is disrupted, eliminating the ability to catalyze reactions. This can be caused by environmental factors such as temperature and pH, which may be outside the optimal range for a given enzyme. In some cases, enzyme denaturation is reversible, allowing the enzyme to regain activity.
One way that environmental pH can impact enzyme activity is through the disruption of hydrogen bonds. These bonds are important for maintaining the three-dimensional structure of the enzyme.
The relative concentrations of substrates and products also play a role in determining the efficiency of an enzymatic reaction. If the concentration of the substrate is too low, the enzyme may not have enough molecules to react with, and the reaction will proceed more slowly. On the other hand, if the concentration of the product is too high, it can inhibit the enzyme's activity by occupying the active site, preventing the substrate from binding.
Higher temperatures can increase the speed of movement of molecules in a solution, which can lead to an increase in the rate of an enzymatic reaction. This is because increased movement increases the frequency of collisions between enzymes and substrates, making the reaction more likely to occur. 🥵
Competitive inhibitors are molecules that can bind to the active site of an enzyme, blocking the substrate from binding. These inhibitors can bind reversibly, meaning they can dissociate from the enzyme, or irreversibly, meaning they bind permanently. Noncompetitive inhibitors, on the other hand, bind to allosteric sites on the enzyme, which are not the active site. These inhibitors change the activity of the enzyme by causing it to change shape, and as a result, the substrate can no longer bind to the active site.
3.4 Energy
All living systems require a constant input of energy in order to maintain their highly ordered state and to power cellular processes. This is because life requires a highly ordered system, and the second law of thermodynamics dictates that in any closed system, entropy, or the measure of disorder, will always increase over time.
Cellular processes that release energy, such as metabolism, are often coupled with processes that require energy, such as the synthesis of molecules or the pumping of ions across a membrane. This allows the organism to harness the energy released by one process to power another.
If the input of energy into a system drops below the energy loss, or if the flow of energy is disrupted, the system will become disordered and will eventually reach a state of equilibrium, resulting in death.

Source: ThoughtCo
In biological systems, energy-related pathways are often sequential, with the product of one reaction serving as the reactant for the next step in the pathway. This allows for a more controlled and efficient transfer of energy, as the energy released by one reaction can be used to drive the next. This can lead to the production of more energy than if the reactions were to occur independently. This is the basis of the electron transport chain (ETC), as we will discuss later!
3.5 Photosynthesis
Organisms capture and store energy from a variety of sources for use in biological processes. One of the most important ways that organisms capture energy is through photosynthesis, which involves the capture of energy from the sun and the production of sugars. 🍭
Photosynthesis first evolved in prokaryotic organisms, which are single-celled organisms that lack a nucleus and other membrane-bound organelles. There is scientific evidence to support the claim that prokaryotic (cyanobacterial) photosynthesis was responsible for the production of an oxygenated atmosphere on Earth. These prokaryotic photosynthetic pathways were the foundation for the development of eukaryotic photosynthesis, which occurs in organisms with a nucleus and other membrane-bound organelles.
The light-dependent reactions of photosynthesis in eukaryotes involve a series of coordinated reaction pathways that capture energy present in light and convert it into ATP and NADPH, which are used to power the production of organic molecules.

Source: Quizlet
During photosynthesis, chlorophylls absorb energy from light, boosting electrons to a higher energy level in photosystems I and II. These photosystems are embedded in the internal membranes of chloroplasts, which are the organelles responsible for photosynthesis in plants and algae. They are connected by the transfer of higher energy electrons through an electron transport chain (ETC). The ETC generates a proton gradient that is used to produce ATP, which is the primary source of energy for cellular processes in most organisms. ⚡
When electrons are transferred between molecules in a sequence of reactions as they pass through the electron transport chain (ETC), an electrochemical gradient of protons (hydrogen ions) is established across the internal membrane of the cell.
The proton gradient is used to synthesize ATP from ADP and inorganic phosphate via ATP synthase, an enzyme that is embedded in the membrane. ATP synthase uses the energy from the proton gradient to power the synthesis of ATP, which is the primary source of energy for most cellular processes. ➕
The energy captured in the light reactions of photosynthesis and transferred to ATP and NADPH powers the production of carbohydrates from carbon dioxide in the Calvin cycle, which occurs in the stroma of the chloroplast. The Calvin cycle is a series of enzyme-catalyzed reactions that use the energy from ATP and NADPH to fix carbon dioxide into organic molecules, such as glucose.
3.6 Cellular Respiration and Fermentation
Fermentation and cellular respiration are two processes that use energy from biological macromolecules to produce ATP. These processes are characteristic of all forms of life and play a vital role in the production of energy in cells. Fermentation is an anaerobic process that occurs in the absence of oxygen and results in the production of ATP through the conversion of organic molecules into simpler products, such as lactate or ethanol. 🍺
Cellular respiration is an aerobic process that occurs in the presence of oxygen and involves a series of coordinated enzyme-catalyzed reactions that capture energy from biological macromolecules, such as glucose. In eukaryotes, cellular respiration occurs in the mitochondria, which are the organelles responsible for energy production in the cell. The energy from cellular respiration is used to synthesize ATP.
In cellular respiration, electrons delivered by NADH and FADH are passed to a series of electron acceptors as they move toward the terminal electron acceptor, oxygen. In photosynthesis, the terminal electron acceptor is NADP+. Aerobic prokaryotes use oxygen as a terminal electron acceptor, while anaerobic prokaryotes use other molecules.
The flow of protons back through membrane-bound ATP synthase drives the formation of ATP from ADP and inorganic phosphate. This process is known as oxidative phosphorylation in cellular respiration and photophosphorylation in photosynthesis. In cellular respiration, decoupling oxidative phosphorylation from electron transport generates heat. This heat can be used by endothermic organisms to regulate body temperature. 🌡

Source: Science Facts
Glycolysis is a biochemical pathway that releases energy from glucose to form ATP from ADP and inorganic phosphate, NADH from NAD+, and pyruvate. This process occurs in the cytosol of cells and is the first step in the breakdown of glucose for energy.
Pyruvate, the end product of glycolysis, is then transported from the cytosol to the mitochondrion, where it undergoes further oxidation. In the mitochondrion, the Krebs cycle, also known as the citric acid cycle, occurs.
The Krebs cycle is a series of enzyme-catalyzed reactions that release energy from organic intermediates, produce carbon dioxide as a waste product, synthesize ATP from ADP and inorganic phosphate, and transfer electrons to the coenzymes NADH and FADH2.
The electrons extracted in glycolysis and the Krebs cycle reactions are then transferred by NADH and FADH2 to the electron transport chain (ETC) in the inner mitochondrial membrane. When electrons are transferred between molecules in a sequence of reactions as they pass through the ETC, an electrochemical gradient of protons (hydrogen ions) across the inner mitochondrial membrane is established. 😁
3.7 Fitness
Variation at the molecular level allows organisms to respond to a variety of environmental stimuli and can provide a greater ability to survive and/or reproduce in different environments. This variation can occur in the number and types of molecules within cells, and it is an important factor in the adaptability of organisms to their surroundings. 💪
Major Formulas
- Cellular Respiration: C6H12O6 + O2 → H2O + CO2
- Photosynthesis: H2O + CO2 → C6H12O6 + O2
- Other key components
- Electron carriers (NADH, FADH2, NADPH)
- Electron carriers are responsible for carrying electrons in the form of a hydrogen ion. They drop these electrons either at the electron transport chain in order to make ATP or in the Calvin Cycle to contribute to bond formation.
- ATP Synthase
- ATP synthase is the enzyme responsible for synthesizing ATP. Remember that enzymes end in -ase. This enzyme harnesses the power of chemiosmosis in order to phosphorylate ADP into ATP.
- Electron carriers (NADH, FADH2, NADPH)

© 2024 Fiveable Inc. All rights reserved.