Browse By Unit
Jed Quiaoit
Jed Quiaoit
In chemical reactions, the transformation of reactants into products often involves multiple intermediate steps, known as elementary reactions. These elementary reactions can be combined to describe the overall reaction through a chemical equation, which shows the reactants, products, and their respective stoichiometric coefficients.
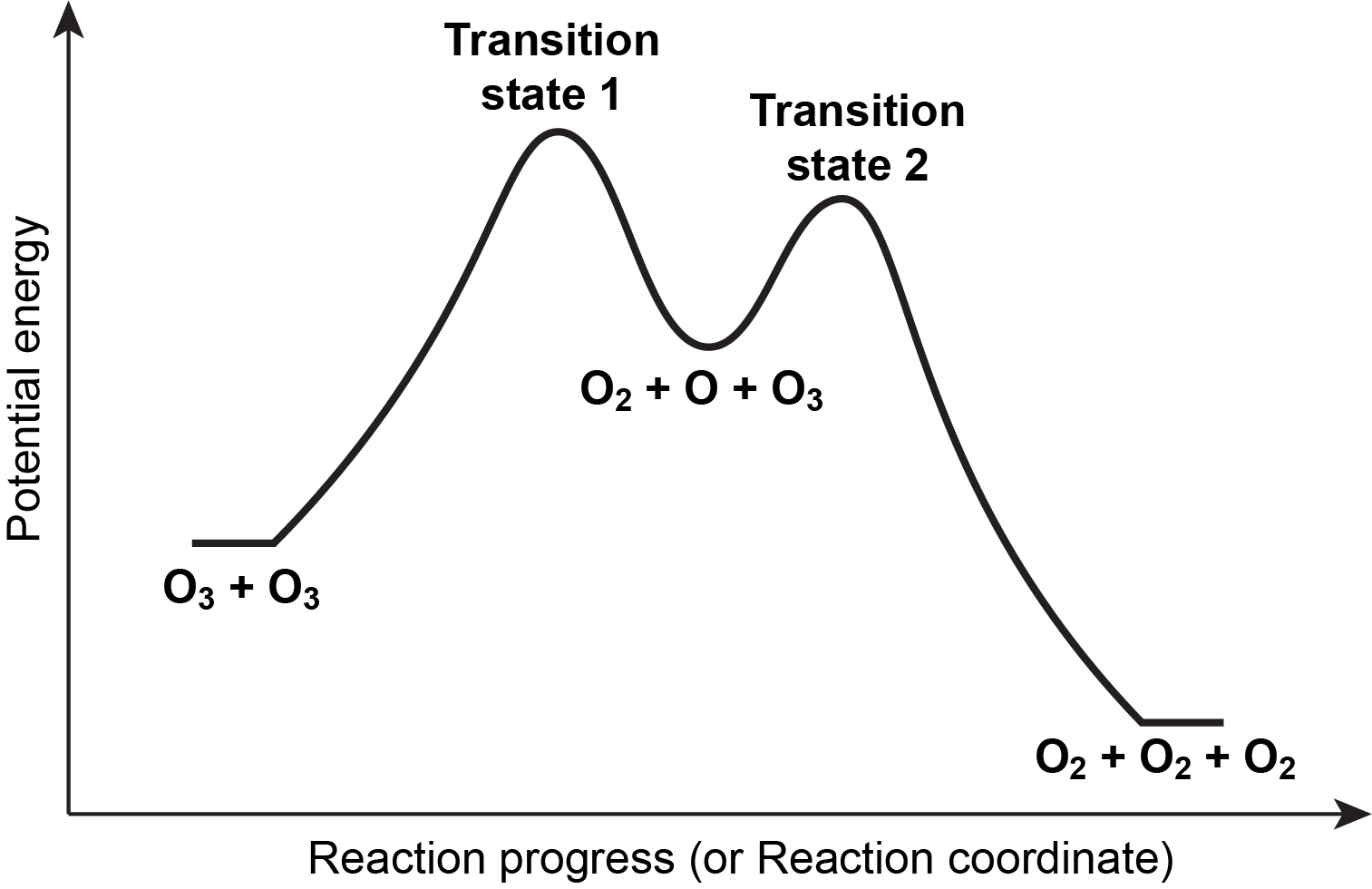
Source: University of Wisconsin
The chemical equation provides a compact representation of the reaction and allows for predictions about the yield and kinetics of the reaction based on the individual rates of the elementary reactions. 📞
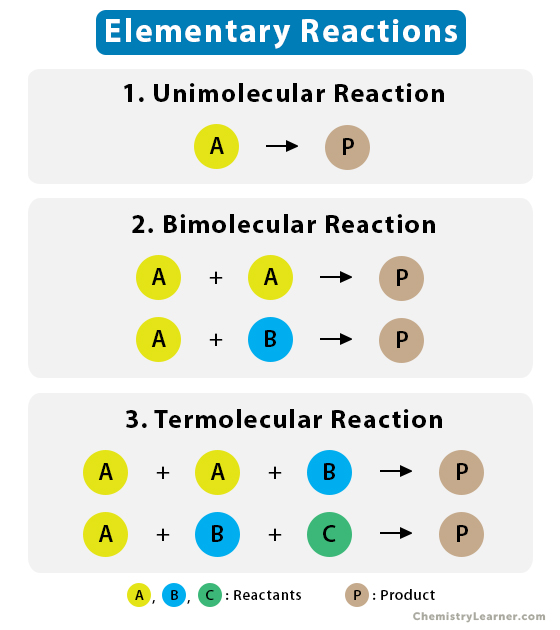
Source: Chemistry Learner
Constructing Reaction Energy Profiles
As alluded to above, a reaction energy profile is a graphical representation of the activation energy and overall energy change in a multistep reaction. It typically shows the potential energy of the reactants and products relative to the reaction coordinate on the y-axis and the reaction progress on the x-axis. The following steps can be taken to represent the activation energy and overall energy change in a multistep reaction with a reaction energy profile: 📦
- Plot the reactants and products: Start by plotting the potential energy of the reactants and products as the starting and ending points of the reaction, respectively.
- Add transition state(s): If the reaction involves a transition state, add a point on the energy profile to represent it. The transition state is the highest energy point along the reaction coordinate, representing the maximum energy barrier that the reactants must overcome to reach the products.
- Plot the energy change: Connect the reactants to the transition state with a curved arrow to represent the activation energy, and connect the transition state to the products with a straight arrow to represent the overall energy change.
- Label the activation energy and overall energy change: Label the activation energy as ΔE₁ and the overall energy change as ΔE₂, and use appropriate units, such as joules or kilojoules. The reaction energy profile provides a visual representation of the energy changes involved in a multistep reaction, allowing for a better understanding of the energetics of the reaction and the role of the activation energy in determining the rate and outcome of the reaction.
Having a comprehensive understanding of the energy changes that occur during each of the elementary reactions in a mechanism is crucial for constructing a reaction energy profile for a multistep reaction.
Reactants, Intermediates, and Products
By having knowledge of the energetics of each elementary reaction in the mechanism, one can determine the highest energy barrier or transition state, the activation energy required to overcome this barrier, and the overall energy change that occurs during the reaction. This information can then be incorporated into the energy profile, which allows for a better understanding of the energetics of the reaction and the factors that influence its rate and outcome. ⚡
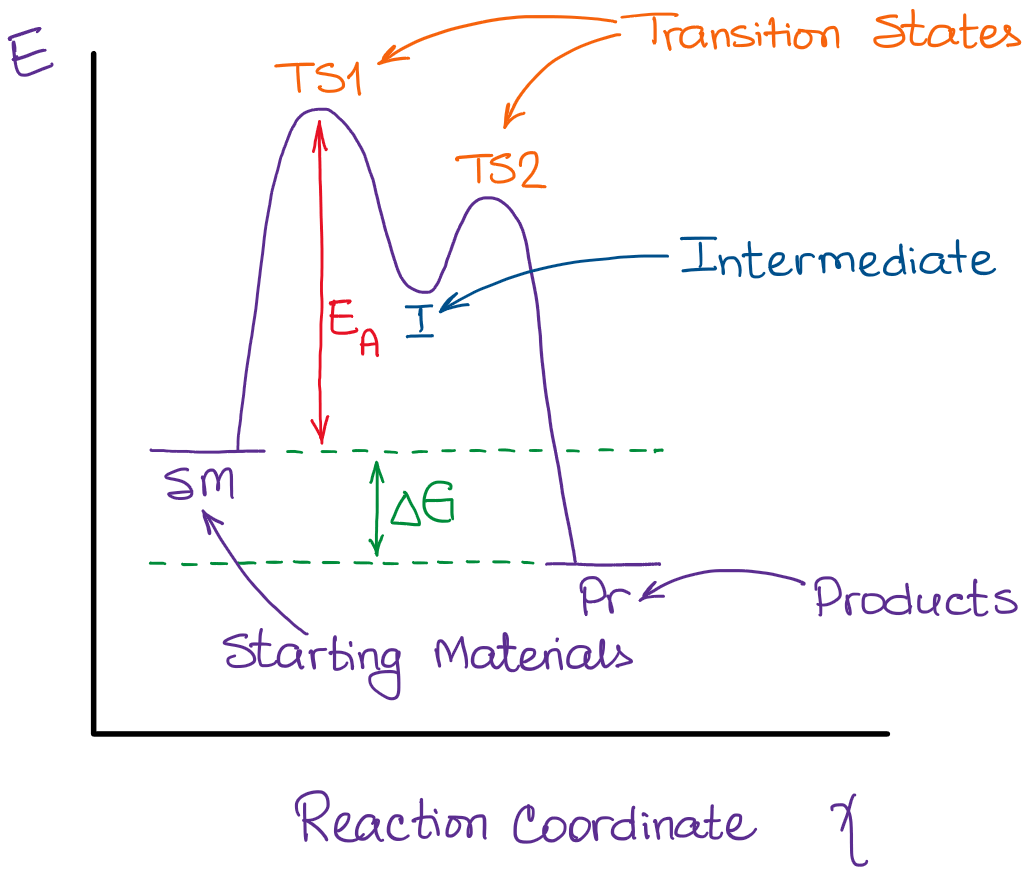
Reactants are the starting substances that react to form new substances. They are written on the left-hand side of a chemical equation.
Intermediates are species that are formed during the reaction and then go on to react further to form the final products. They are not directly involved in the overall reaction and are usually not present at the beginning or end of the reaction.
Products are the final substances formed after the reaction has taken place. They are written on the right-hand side of a chemical equation.
An example of a reaction involving reactants, intermediates, and products is the reaction between hydrogen gas (H₂) and nitrogen gas (N₂) to form ammonia (NH₃) through the Haber process:
- Reactants: H₂(g) + N₂(g)
- Intermediate: N₂H₃⁺
- Products: NH₃(g) In this reaction, the reactants H₂ and N₂ react to form the intermediate N₂H₃⁺, which then reacts further to form the final product NH₃! ⚛️
<< Hide Menu
Jed Quiaoit
Jed Quiaoit
In chemical reactions, the transformation of reactants into products often involves multiple intermediate steps, known as elementary reactions. These elementary reactions can be combined to describe the overall reaction through a chemical equation, which shows the reactants, products, and their respective stoichiometric coefficients.

Source: University of Wisconsin
The chemical equation provides a compact representation of the reaction and allows for predictions about the yield and kinetics of the reaction based on the individual rates of the elementary reactions. 📞

Source: Chemistry Learner
Constructing Reaction Energy Profiles
As alluded to above, a reaction energy profile is a graphical representation of the activation energy and overall energy change in a multistep reaction. It typically shows the potential energy of the reactants and products relative to the reaction coordinate on the y-axis and the reaction progress on the x-axis. The following steps can be taken to represent the activation energy and overall energy change in a multistep reaction with a reaction energy profile: 📦
- Plot the reactants and products: Start by plotting the potential energy of the reactants and products as the starting and ending points of the reaction, respectively.
- Add transition state(s): If the reaction involves a transition state, add a point on the energy profile to represent it. The transition state is the highest energy point along the reaction coordinate, representing the maximum energy barrier that the reactants must overcome to reach the products.
- Plot the energy change: Connect the reactants to the transition state with a curved arrow to represent the activation energy, and connect the transition state to the products with a straight arrow to represent the overall energy change.
- Label the activation energy and overall energy change: Label the activation energy as ΔE₁ and the overall energy change as ΔE₂, and use appropriate units, such as joules or kilojoules. The reaction energy profile provides a visual representation of the energy changes involved in a multistep reaction, allowing for a better understanding of the energetics of the reaction and the role of the activation energy in determining the rate and outcome of the reaction.
Having a comprehensive understanding of the energy changes that occur during each of the elementary reactions in a mechanism is crucial for constructing a reaction energy profile for a multistep reaction.
Reactants, Intermediates, and Products
By having knowledge of the energetics of each elementary reaction in the mechanism, one can determine the highest energy barrier or transition state, the activation energy required to overcome this barrier, and the overall energy change that occurs during the reaction. This information can then be incorporated into the energy profile, which allows for a better understanding of the energetics of the reaction and the factors that influence its rate and outcome. ⚡

Reactants are the starting substances that react to form new substances. They are written on the left-hand side of a chemical equation.
Intermediates are species that are formed during the reaction and then go on to react further to form the final products. They are not directly involved in the overall reaction and are usually not present at the beginning or end of the reaction.
Products are the final substances formed after the reaction has taken place. They are written on the right-hand side of a chemical equation.
An example of a reaction involving reactants, intermediates, and products is the reaction between hydrogen gas (H₂) and nitrogen gas (N₂) to form ammonia (NH₃) through the Haber process:
- Reactants: H₂(g) + N₂(g)
- Intermediate: N₂H₃⁺
- Products: NH₃(g) In this reaction, the reactants H₂ and N₂ react to form the intermediate N₂H₃⁺, which then reacts further to form the final product NH₃! ⚛️

© 2025 Fiveable Inc. All rights reserved.