Browse By Unit
Jillian Holbrook
Jillian Holbrook
Thermodynamically Unfavorable Reactions
For most of this unit, we have discussed thermodynamically favorable reactions, reactions for which ΔG° < 0 and K > 1. However, this section looks at thermodynamically unfavorable reactions. Thermodynamically unfavorable reactions do not occur spontaneously and, therefore, will not happen without any sort of external energy source.
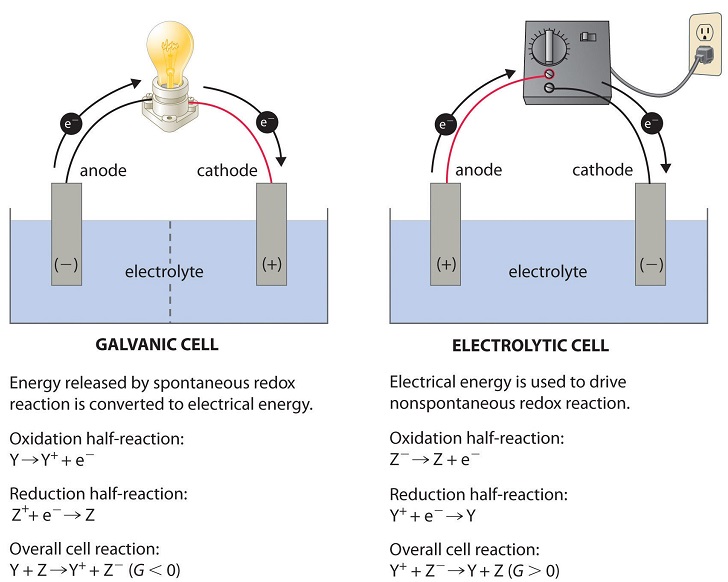
External energy sources can help nonspontaneous processes run. A common source of energy for spurring nonspontaneous processes is electricity. By using electrical energy, nonspontaneous redox reactions can take place. For example, a battery can be connected to an electrolytic cell (a topic we will discuss in Unit 9.7) to “push” electrons from a negatively charged ion to a positively charged ion in a reaction of the form Y+ + Z- → Y + Z (assuming Y + Z → Y+ + Z- is spontaneous). These cells are also similar to the way a battery is charged! 🔋
Coupled Reactions Explained
Another way to make nonspontaneous reactions spontaneous is through the use of coupled reactions. Coupled reactions are a combination of a nonspontaneous reaction and a spontaneous reaction that have a common intermediate. Recall from kinetics that an intermediate is a product of one part of a mechanism and a reactant in the next. For example, in the following mechanism, O is an intermediate:
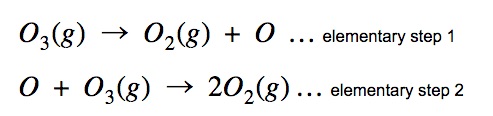
By using reactions with common intermediates, mechanisms for new reactions can form. By adding together these elementary steps, a new reaction results, which is spontaneous. An example of the following is clear in the subsequent reaction:
Cu2S → 2Cu + S (ΔG° = 86.2 kJ)
We see that this reaction is nonspontaneous and requires some external energy to occur. However, we can also find a spontaneous reaction with either Cu or S as a reactant that we can add to the reaction to create a spontaneous process overall (ΔG° < 0).
For example, let’s use the reaction:
S + O2 → SO2 (ΔG° = -300.1 kJ)
By adding these reactions together we find the following:
Cu2S → 2Cu + S (ΔG° = 86.2 kJ)
S + O2 → SO2 (ΔG° = -300.1 kJ)
Cu2S + O2 → 2Cu + SO2 (ΔG° = 86.2 + (-300.1) = -213.9)
As we see, by adding these reactions together, we can use the spontaneous process of sulfur and oxygen forming sulfur dioxide to couple the reactions and form a process that is spontaneous overall.
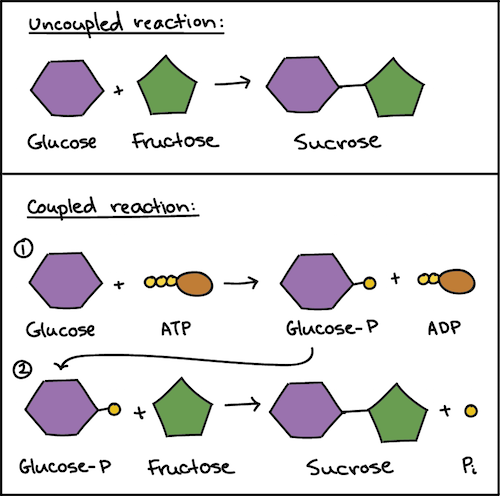
This concept finds many applications in biology, including the conversion of ATP to ADP in biological systems. The example below comes from organic chemistry but is another example of reaction coupling. Do not worry if you are unfamiliar with the terms ATP and ADP or what all the hexagons and pentagons mean:
Practice Problem
Given the following reactions and thermodynamic data, calculate the ΔG° value for the reaction Fe2O3 + 3CO → 2Fe + 3CO2:
Fe2O3 → 2Fe + 3/2O2 (ΔG° = 742.2 kJ)
CO + 1/2O2 → CO2 (ΔG° = -283.5 kJ)
We can use reaction coupling to solve this problem because the two reactions have a common intermediate of O2. However, we first want to multiply the bottom reaction by three to make the O2s cancel out. When we do so, we also multiply ΔG° by the same factor.
Fe2O3 → 2Fe + 3/2O2 (ΔG° = 742.2 kJ)
3CO + 3/2O2 → 3CO2 (ΔG° = -283.5 * 3 kJ = -850.5 kJ)
Fe2O3 + 3CO + 3/2O2 → 2Fe + 3CO2 + 3/2O2 (ΔG° = 742.2 kJ + -850.5 kJ = -108.3 kJ)
Fe2O3 + 3CO+ → 2Fe + 3CO2 (ΔG° = -108.3 kJ)
<< Hide Menu
Jillian Holbrook
Jillian Holbrook
Thermodynamically Unfavorable Reactions
For most of this unit, we have discussed thermodynamically favorable reactions, reactions for which ΔG° < 0 and K > 1. However, this section looks at thermodynamically unfavorable reactions. Thermodynamically unfavorable reactions do not occur spontaneously and, therefore, will not happen without any sort of external energy source.
External energy sources can help nonspontaneous processes run. A common source of energy for spurring nonspontaneous processes is electricity. By using electrical energy, nonspontaneous redox reactions can take place. For example, a battery can be connected to an electrolytic cell (a topic we will discuss in Unit 9.7) to “push” electrons from a negatively charged ion to a positively charged ion in a reaction of the form Y+ + Z- → Y + Z (assuming Y + Z → Y+ + Z- is spontaneous). These cells are also similar to the way a battery is charged! 🔋

Coupled Reactions Explained
Another way to make nonspontaneous reactions spontaneous is through the use of coupled reactions. Coupled reactions are a combination of a nonspontaneous reaction and a spontaneous reaction that have a common intermediate. Recall from kinetics that an intermediate is a product of one part of a mechanism and a reactant in the next. For example, in the following mechanism, O is an intermediate:
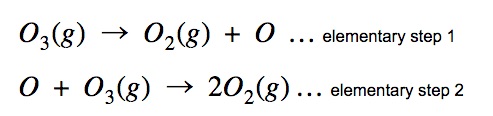
By using reactions with common intermediates, mechanisms for new reactions can form. By adding together these elementary steps, a new reaction results, which is spontaneous. An example of the following is clear in the subsequent reaction:
Cu2S → 2Cu + S (ΔG° = 86.2 kJ)
We see that this reaction is nonspontaneous and requires some external energy to occur. However, we can also find a spontaneous reaction with either Cu or S as a reactant that we can add to the reaction to create a spontaneous process overall (ΔG° < 0).
For example, let’s use the reaction:
S + O2 → SO2 (ΔG° = -300.1 kJ)
By adding these reactions together we find the following:
Cu2S → 2Cu + S (ΔG° = 86.2 kJ)
S + O2 → SO2 (ΔG° = -300.1 kJ)
Cu2S + O2 → 2Cu + SO2 (ΔG° = 86.2 + (-300.1) = -213.9)
As we see, by adding these reactions together, we can use the spontaneous process of sulfur and oxygen forming sulfur dioxide to couple the reactions and form a process that is spontaneous overall.
This concept finds many applications in biology, including the conversion of ATP to ADP in biological systems. The example below comes from organic chemistry but is another example of reaction coupling. Do not worry if you are unfamiliar with the terms ATP and ADP or what all the hexagons and pentagons mean:

Practice Problem
Given the following reactions and thermodynamic data, calculate the ΔG° value for the reaction Fe2O3 + 3CO → 2Fe + 3CO2:
Fe2O3 → 2Fe + 3/2O2 (ΔG° = 742.2 kJ)
CO + 1/2O2 → CO2 (ΔG° = -283.5 kJ)
We can use reaction coupling to solve this problem because the two reactions have a common intermediate of O2. However, we first want to multiply the bottom reaction by three to make the O2s cancel out. When we do so, we also multiply ΔG° by the same factor.
Fe2O3 → 2Fe + 3/2O2 (ΔG° = 742.2 kJ)
3CO + 3/2O2 → 3CO2 (ΔG° = -283.5 * 3 kJ = -850.5 kJ)
Fe2O3 + 3CO + 3/2O2 → 2Fe + 3CO2 + 3/2O2 (ΔG° = 742.2 kJ + -850.5 kJ = -108.3 kJ)
Fe2O3 + 3CO+ → 2Fe + 3CO2 (ΔG° = -108.3 kJ)

© 2025 Fiveable Inc. All rights reserved.