Browse By Unit
Unit 7 Overview: Atmospheric Pollution
4 min read•june 18, 2024
Jenni MacLean
Krish Gupta
Jenni MacLean
Krish Gupta
7.0: Intro to Unit 7

Image from Pixabay
Welcome to unit 7! In this unit, we will look at all things air pollution 💨 and its impact. Air is a common good that moves freely from one area to another, and pollution generated in one region can impact people far away. Subsequently, there is a need for political reform and regulations to protect the public. Legislation like the Clean Air Act regulates emissions of pollutants and puts public health first. So let's jump in and take a look at the types of atmospheric pollution, how they are classified and who they are impacting.
Introduction to Air Pollution
So, what even causes air pollution? Well, air pollution mostly comes from burning things🔥. Any time we burn or combust something, there are byproducts released into the air. More specifically, there are impurities in fuel that are released when we do things like drive our cars. 🚗
These chemicals fit into one of two major categories: primary air pollutants and secondary air pollutants.
Primary air pollutants are released directly from their sources into the atmosphere.
Examples:
- Carbon Monoxide (CO)
- Nitrogen dioxide (NO2)
- Sulfur dioxide (SO2)
- Particulate matter
- Lead
Secondary air pollutants form when primary air pollutants react with chemicals in the atmosphere.
Example:
- Tropospheric Ozone (O3)
Photochemical Smog

Image from Pixabay
This is one of those subjects that intimidate students😨, but don't let it! Smog is an easy enough concept to understand with just a little bit of chemistry sprinkled in. Just think about it this way: smog is created by cars 🚙 and sunlight🌞.
Remember that cities with a lot of cars and warmer climates are more prone to smog, especially if large mountain ranges surround them. These ranges will trap the air, and the smog often settles and accumulates for long periods until a storm blows it away. Great examples of this would be Los Angeles and Mexico City.
So what is smog? Photochemical smog is created when nitrogen oxides and volatile organic hydrocarbons react with heat and sunlight.
Nitrogen dioxide (NO2) is a primary pollutant that is generated by the combustion of fossil fuels. Nitric oxide (NO) oxidizes to form nitrogen dioxide (NO2) in the atmosphere. (When referencing both together, we notate nitrogen oxides as NOx).
Tropospheric ozone (O3) is a secondary pollutant. Sunlight interacts with nitrogen oxides (NOx) and hydrocarbons to form O3. This tropospheric ozone is a major component of smog creation.
Thermal Inversion

Image from Wikipedia.
Any deviation from an average heat gradient in the atmosphere is considered a Thermal Inversion. The usual pattern is when the warmest air next to the land gets cooler and further away from Earth. An inversion occurs when a layer of warm air sits on top of a colder layer of polluted air. The cooler polluted air is trapped and prevented from moving.
Thermal inversions are much more common in cities in a bowl or valley, which can trap the polluted air for an extended period of time—starting to sound familiar? Once again, good example cities for air pollution are Los Angeles and Mexico City. The geography and large population of people driving cars create a pocket of very polluted air. It often takes a storm to push the air away from the city. The image above shows how the contaminated air is visible in these ‘bad air days.’
Indoor Air Pollutants
When we think of air pollution, we get a mental image like the one above or a smokestack from a factory, but we don't tend to think about the air around us as we watch tv or sit in our classroom!
Indoor air pollutants can be even more detrimental to our health because we spend so much of our time inside buildings without circulating in new air from outside (particularly in regions that are very cold or hot because we have the windows closed).
Here is a list of indoor air pollutants and their sources. It's scary to think that you might be more at risk from contaminants from your couch than a factory😟.
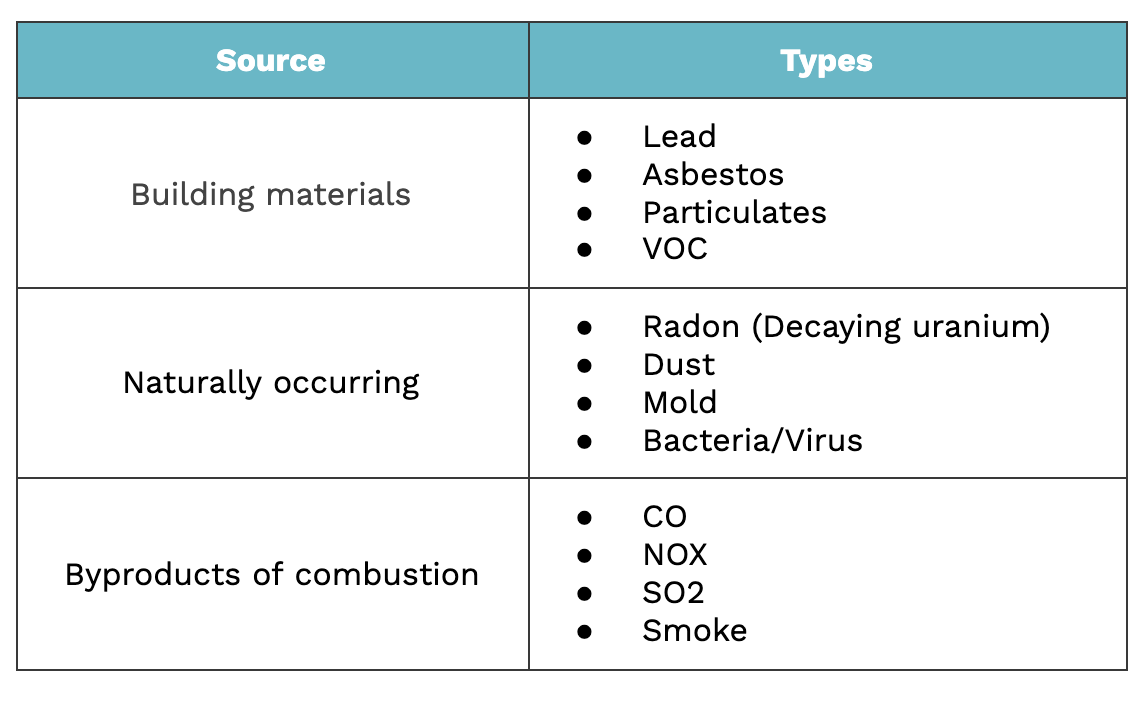
Reduction of Air Pollutants
So what are we going to do about it? Once we identify sources of pollution, humans have made significant steps to reduce their levels. In general, reduction comes from regulation and conservation. When we burn fewer fossil fuels, we produce fewer pollutants. Movements into green energy sources, fuel-efficient vehicles, and commuting less can drastically improve air quality. The pandemic and work-from-home culture has created significantly cleaner air in large cities!
Here are some regulations for air quality and atmospheric pollutants:
Clean Air Act: Set emission standards for cars and limits the release of pollutants.
Kyoto Protocol: Sets greenhouse gas emissions reduction standards. Not joined by all countries and not highly effective.
Montreal Protocol: Phases out the use of CFCs was widely joined and highly effective.
<< Hide Menu
Unit 7 Overview: Atmospheric Pollution
4 min read•june 18, 2024
Jenni MacLean
Krish Gupta
Jenni MacLean
Krish Gupta
7.0: Intro to Unit 7

Image from Pixabay
Welcome to unit 7! In this unit, we will look at all things air pollution 💨 and its impact. Air is a common good that moves freely from one area to another, and pollution generated in one region can impact people far away. Subsequently, there is a need for political reform and regulations to protect the public. Legislation like the Clean Air Act regulates emissions of pollutants and puts public health first. So let's jump in and take a look at the types of atmospheric pollution, how they are classified and who they are impacting.
Introduction to Air Pollution
So, what even causes air pollution? Well, air pollution mostly comes from burning things🔥. Any time we burn or combust something, there are byproducts released into the air. More specifically, there are impurities in fuel that are released when we do things like drive our cars. 🚗
These chemicals fit into one of two major categories: primary air pollutants and secondary air pollutants.
Primary air pollutants are released directly from their sources into the atmosphere.
Examples:
- Carbon Monoxide (CO)
- Nitrogen dioxide (NO2)
- Sulfur dioxide (SO2)
- Particulate matter
- Lead
Secondary air pollutants form when primary air pollutants react with chemicals in the atmosphere.
Example:
- Tropospheric Ozone (O3)
Photochemical Smog

Image from Pixabay
This is one of those subjects that intimidate students😨, but don't let it! Smog is an easy enough concept to understand with just a little bit of chemistry sprinkled in. Just think about it this way: smog is created by cars 🚙 and sunlight🌞.
Remember that cities with a lot of cars and warmer climates are more prone to smog, especially if large mountain ranges surround them. These ranges will trap the air, and the smog often settles and accumulates for long periods until a storm blows it away. Great examples of this would be Los Angeles and Mexico City.
So what is smog? Photochemical smog is created when nitrogen oxides and volatile organic hydrocarbons react with heat and sunlight.
Nitrogen dioxide (NO2) is a primary pollutant that is generated by the combustion of fossil fuels. Nitric oxide (NO) oxidizes to form nitrogen dioxide (NO2) in the atmosphere. (When referencing both together, we notate nitrogen oxides as NOx).
Tropospheric ozone (O3) is a secondary pollutant. Sunlight interacts with nitrogen oxides (NOx) and hydrocarbons to form O3. This tropospheric ozone is a major component of smog creation.
Thermal Inversion

Image from Wikipedia.
Any deviation from an average heat gradient in the atmosphere is considered a Thermal Inversion. The usual pattern is when the warmest air next to the land gets cooler and further away from Earth. An inversion occurs when a layer of warm air sits on top of a colder layer of polluted air. The cooler polluted air is trapped and prevented from moving.
Thermal inversions are much more common in cities in a bowl or valley, which can trap the polluted air for an extended period of time—starting to sound familiar? Once again, good example cities for air pollution are Los Angeles and Mexico City. The geography and large population of people driving cars create a pocket of very polluted air. It often takes a storm to push the air away from the city. The image above shows how the contaminated air is visible in these ‘bad air days.’
Indoor Air Pollutants
When we think of air pollution, we get a mental image like the one above or a smokestack from a factory, but we don't tend to think about the air around us as we watch tv or sit in our classroom!
Indoor air pollutants can be even more detrimental to our health because we spend so much of our time inside buildings without circulating in new air from outside (particularly in regions that are very cold or hot because we have the windows closed).
Here is a list of indoor air pollutants and their sources. It's scary to think that you might be more at risk from contaminants from your couch than a factory😟.

Reduction of Air Pollutants
So what are we going to do about it? Once we identify sources of pollution, humans have made significant steps to reduce their levels. In general, reduction comes from regulation and conservation. When we burn fewer fossil fuels, we produce fewer pollutants. Movements into green energy sources, fuel-efficient vehicles, and commuting less can drastically improve air quality. The pandemic and work-from-home culture has created significantly cleaner air in large cities!
Here are some regulations for air quality and atmospheric pollutants:
Clean Air Act: Set emission standards for cars and limits the release of pollutants.
Kyoto Protocol: Sets greenhouse gas emissions reduction standards. Not joined by all countries and not highly effective.
Montreal Protocol: Phases out the use of CFCs was widely joined and highly effective.

© 2024 Fiveable Inc. All rights reserved.