Browse By Unit
Jenni MacLean
Jenni MacLean
The atmosphere is organized into layers based on density and temperature.
Ozone (O3) in the stratosphere absorbs incoming electromagnetic radiation from the sun. Without the ozone, the UV rays would be able to hit organisms on Earth and cause negative health effects like cancer and cataracts.
Chlorofluorocarbons (CFCs) are a man-made chemical, created as a stable molecule to deliver other substances via aerosol. They were widely used in hairspray and air fresheners. Chlorofluorocarbons (CFCs) are highly stable and are able to travel into the stratosphere without reacting or breaking down. However, they do split apart when hit by incoming stratospheric electromagnetic radiation. Once broken into their components, they are highly reactive and bind with the available oxygen needed to continuously create and heal the ozone layer.
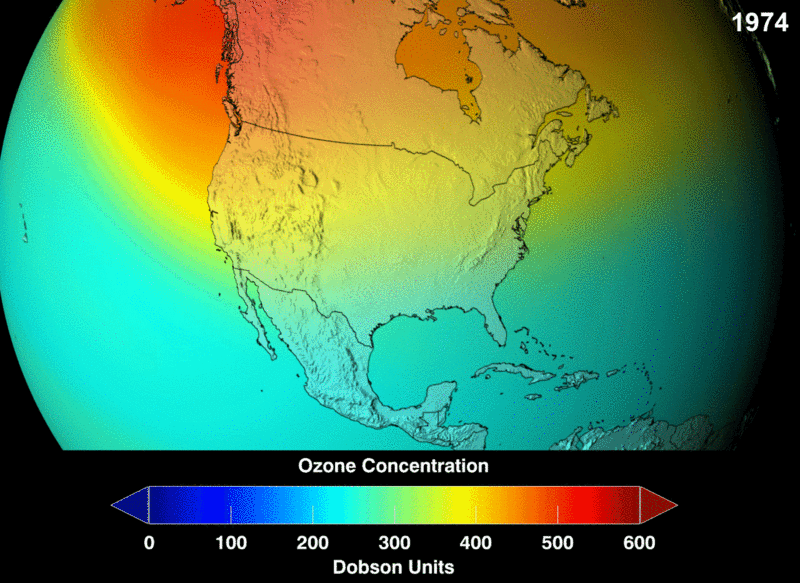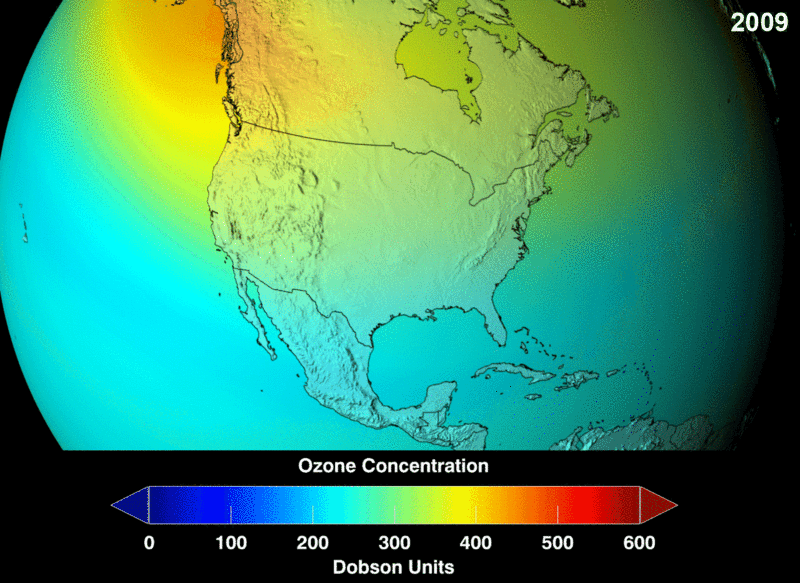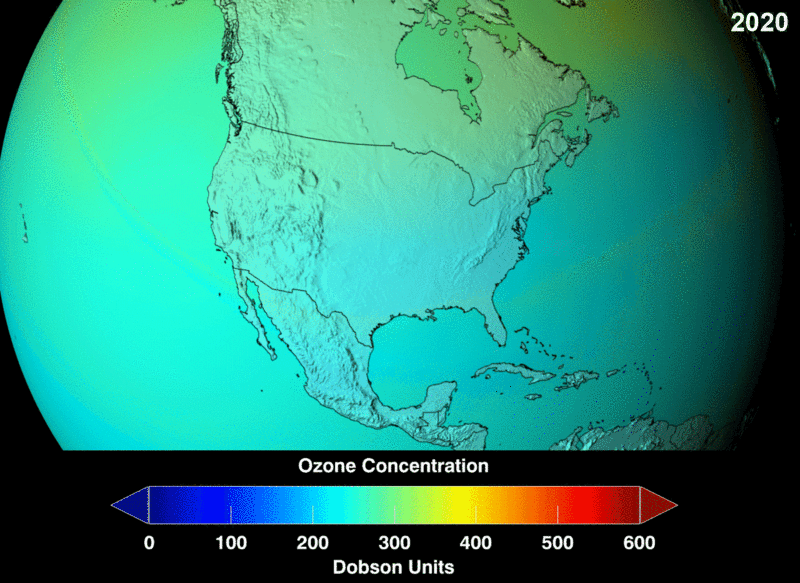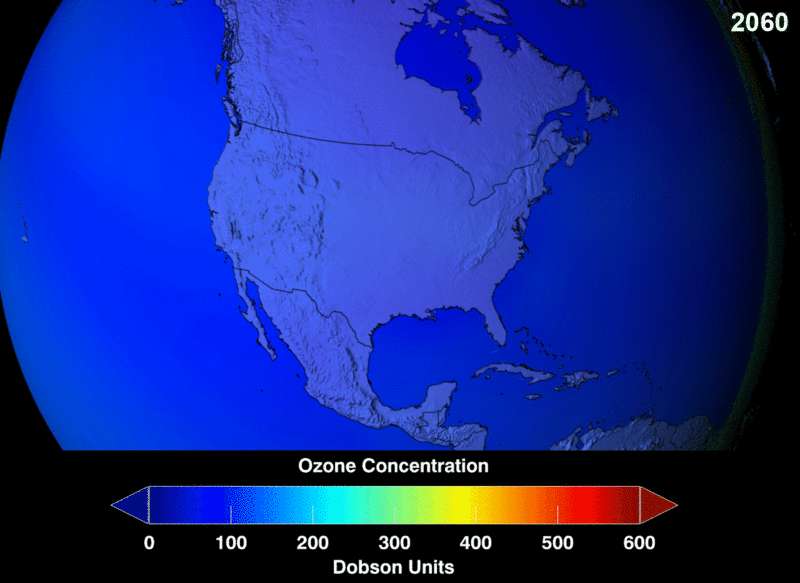
Image Courtesy of Wikimedia
Relevant Chemical Equations
Typical Ozone Cycle
Step 1: O3 + electromagnetic radiation → O + O2
Step 2: O + O2 → O3
The ozone is continuously splitting apart and reforming as it absorbs electromagnetic radiation.
Ozone Depletion with Chlorofluorocarbons (CFCs)
One molecule of Chlorofluorocarbon can continuously bond with and destroy ozone.
-
Step 1: CFCl3 + electromagnetic radiation → Cl + CFCl2 A chlorofluorocarbon is hit by electromagnetic radiation causing it to lose a chlorine atom.
-
Step 2: Cl + O3 → ClO + O2 A chlorine atom removes an oxygen atom from an ozone molecule to make a ClO molecule
-
Step 3: ClO + O3 → Cl· + 2 O2 This ClO can also remove an oxygen atom from another ozone molecule; the chlorine is free to repeat this two-step cycle
🎥 Watch: AP Environmental Streams
<< Hide Menu
Jenni MacLean
Jenni MacLean
The atmosphere is organized into layers based on density and temperature.
Ozone (O3) in the stratosphere absorbs incoming electromagnetic radiation from the sun. Without the ozone, the UV rays would be able to hit organisms on Earth and cause negative health effects like cancer and cataracts.
Chlorofluorocarbons (CFCs) are a man-made chemical, created as a stable molecule to deliver other substances via aerosol. They were widely used in hairspray and air fresheners. Chlorofluorocarbons (CFCs) are highly stable and are able to travel into the stratosphere without reacting or breaking down. However, they do split apart when hit by incoming stratospheric electromagnetic radiation. Once broken into their components, they are highly reactive and bind with the available oxygen needed to continuously create and heal the ozone layer.

Image Courtesy of Wikimedia
Relevant Chemical Equations
Typical Ozone Cycle
Step 1: O3 + electromagnetic radiation → O + O2
Step 2: O + O2 → O3
The ozone is continuously splitting apart and reforming as it absorbs electromagnetic radiation.
Ozone Depletion with Chlorofluorocarbons (CFCs)
One molecule of Chlorofluorocarbon can continuously bond with and destroy ozone.
-
Step 1: CFCl3 + electromagnetic radiation → Cl + CFCl2 A chlorofluorocarbon is hit by electromagnetic radiation causing it to lose a chlorine atom.
-
Step 2: Cl + O3 → ClO + O2 A chlorine atom removes an oxygen atom from an ozone molecule to make a ClO molecule
-
Step 3: ClO + O3 → Cl· + 2 O2 This ClO can also remove an oxygen atom from another ozone molecule; the chlorine is free to repeat this two-step cycle
🎥 Watch: AP Environmental Streams

© 2024 Fiveable Inc. All rights reserved.