Browse By Unit
The Ultimate AP Physics 2 Exam Guide
7 min read•june 18, 2024
Peter Apps
Peter Apps
Introduction
A quick pep talk before we start
You've been (likely) prepping for months for this exam! 🤓 One test can never measure everything you've learned from studying physics. So please, don't let your AP scores define you!
Now that we've gotten that out of the way, let's focus a bit more on the actual exam.
Exam Logistics 🤔
The AP Physics 2 exam (just like almost every other exam) consists of 2 sections: Multiple Choice Questions (MCQs) and Free-Response Questions (FRQs).
In Section 1, you will have 90 minutes to answer 50 MCQs, whereas, in Section 2, you will have 90 minutes to answer 4 FRQs, which are listed below. Each section is weighted at 50% of your exam score, so it's important to prepare for both sections! 📚
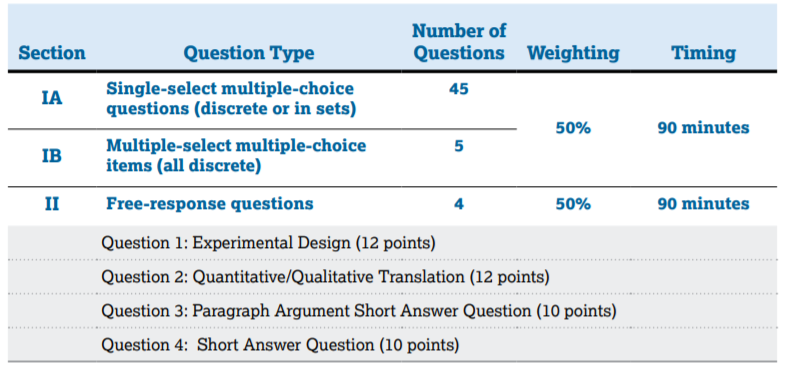
Image Courtesy of AP Physics 2 Course & Exam Description
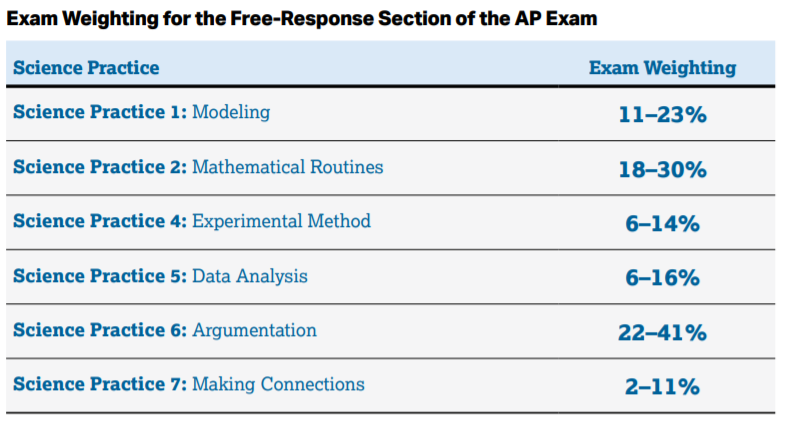
As far as trying to guess what topics will be on the exam, College Board has given us this rough estimate of how the questions will be broken down:
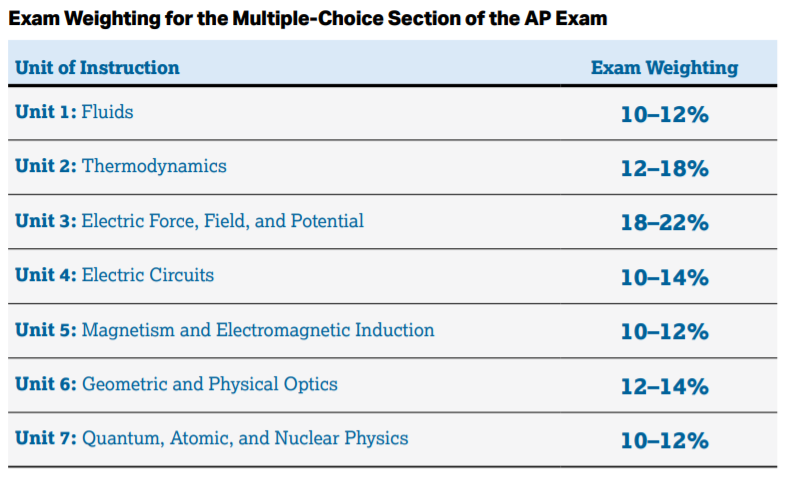
Image Courtesy of AP Physics 2 Course & Exam Description
Section 1: Multiple Choice Questions
This section mostly consists of (you guessed it) Multiple Choice Questions. You're more likely to be asked a question about modeling, argumentation, or mathematically calculating a value than you are to be asked questions about experimental methods, or data analysis.
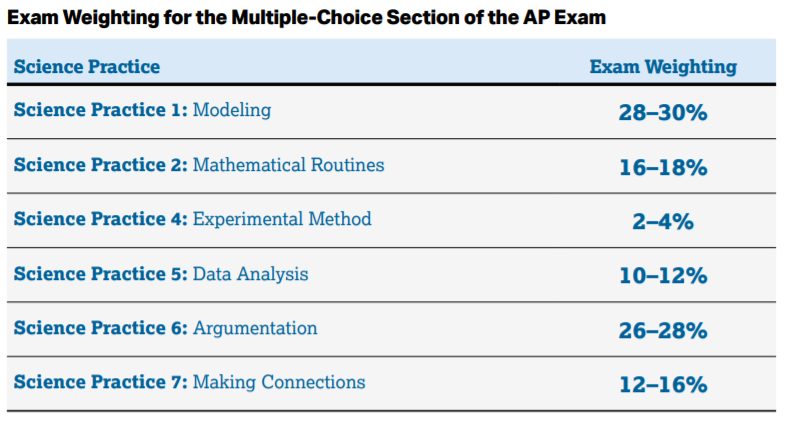
Image Courtesy of AP Physics 2 Course & Exam Description
Strategies For the Multiple Choice Section 💪
- Read the question fully before trying to answer. There's often a tidbit in the image or description that is essential to correctly solve the problem. Don't just skim.
- It's ok to narrow down to 2 choices before guessing. CollegeBoard doesn't expect perfection and a 50% chance of getting the right answer is a lot better than leaving it blank.
- Reference Tables are your friend! Even if it's for something as simple as "What's the unit for charge?" It's a free resource so use it!
- Start with a question you know you'll get right! A little boost of confidence can be the difference between a very difficult test and one that's much easier.
- Watch your time! You've got about 90 seconds to answer each question. Don't feel bad about skipping a harder question and coming back to it if you have time. (Just make sure you don't leave any blanks on that answer sheet!)
Sample MC Questions
All of these questions can be found on the AP Physics 2 Course and Exam Description
Answers (Don't peek!👀)
- The correct answer is C
Kinetic Energy of a gas is proportional to the temperature (K = 3/2*kBT). You can calculate the temperature of the gas at each point using the Ideal Gas Law (PV = nRT). Plugging in for each of the 3 locations, we see that point A and C both have a temperature of (3P0V0) / (nR) while point B has a temperature of (P0V0) / (nR). Therefore A and C have the same temperature which is higher than point B.
- The correct answer is D
Pressure is defined as Force per unit Area. A higher pressure will result in a higher force being applied to the piston. Point C has 3 times the pressure as A & B so it will have a higher force.

Answer (Don't peek!👀)
The correct answer is C. The resistance of the cylinder is proportional to the length and inversely proportional to the cross-sectional area R = pL / A because the area of the cylinder depends on the square of the radius, doubling both the length and the radius will result in 1/2 as much resistance which should double the current.

Answer (Don't peek!👀)
The correct answer is B. Use Bernoulli's continuity equation

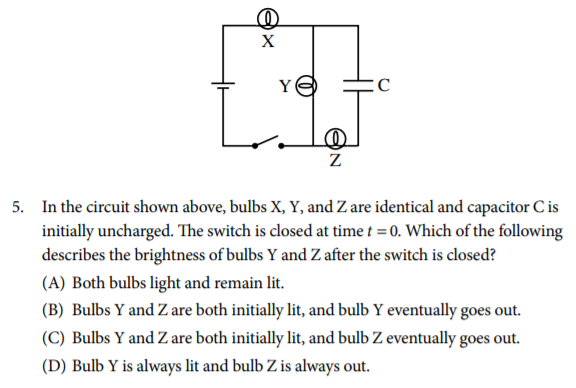
Answer (Don't peek!👀)
The correct answer is C. Bulbs X and Y are connected directly to the battery through the switch and therefore are controlled directly by the switch. Bulb Z relies on current passing through the capacitor to be lit. Initially the uncharged capacitor allows current to flow through it, until the voltage of the capacitor reaches the same voltage as the battery. When this happens there is no flow of current through bulb Z and it goes out.
Section 2: Free-Response Questions
So you've made it through the MCQ, now we've got 90 minutes each to tackle 4 FRQ's. Unlike the MCQs, we know the four question types. There will be an experimental design (12 points), qualitative/quantitative translation (12 points), paragraph argument (10 points), and one additional question (10 points). We're still focusing a lot on theoretical relationships, math routines, and arguments. This is also the section where derivations come into play.
Strategies for the FRQ 🤨
- Pace yourself! Look at the suggested times for each question and try to stick within those recommendations.
- Graphs and drawings are easy points. If you're crunched for time and have to choose between a graph section or a derivation, choose the graph one!
- Reference tables are there for a reason! Use them!
- Remember to BCDA your arguments (Bring in Physics concepts, Cite info from the problem, Draw the info and the physics together, and finally, answer the question)
- Know your vocab! (Check out the list below)
Vocab is fun!
- Calculate: Perform mathematical steps to arrive at a final answer, including algebraic expressions, properly substituted numbers, and correct labeling of units and significant figures. Also phrased as “What is?”
- Compare: Provide a description or explanation of similarities and/or differences.
- Derive: Perform a series of mathematical steps using equations or laws to arrive at a final answer.
- Describe: Provide the relevant characteristics of a specified topic.
- Determine: Make a decision or arrive at a conclusion after reasoning, observation, or applying mathematical routines (calculations).
- Estimate: Roughly calculate numerical quantities, values (greater than, equal to, less than), or signs (negative, positive) of quantities based on experimental evidence or provided data. When making estimations, showing steps in calculations are not required.
- Explain: Provide information about how or why a relationship, process, pattern, position, situation, or outcome occurs, using evidence and/or reasoning to support or qualify a claim. Explain “how” typically requires analyzing the relationship, process, pattern, position, situation, or outcome; whereas, explain “why” typically requires analysis of motivations or reasons for the relationship, process, pattern, position, situation, or outcome.
- Justify: Provide evidence to support, qualify, or defend a claim, and/or provide reasoning to explain how that evidence supports or qualifies the claim.
- Label: Provide labels indicating unit, scale, and/or components in a diagram, graph, model, or representation.
- Plot: Draw data points in a graph using a given scale or indicating the scale and units, demonstrating consistency between different types of representations.
- Sketch/Draw: Create a diagram, graph, representation, or model that illustrates or explains relationships or phenomena, demonstrating consistency between different types of representations. Labels may or may not be required.
- State/Indicate/Circle: Indicate or provide information about a specified topic, without elaboration or explanation. Also phrased as “What…?” or ”Would…?” interrogatory questions.
- Verify: Confirm that the conditions of a scientific definition, law, theorem, or test are met in order to explain why it applies in a given situation. Also, use empirical data, observations, tests, or experiments to prove, confirm, and/or justify a hypothesis.
Sample Free Response Questions
All of these questions can be found on the AP Physics 2 Course and Exam Description
Answers (Don't peek!👀)
A) There are a bunch of different procedures you could choose from. The big idea is to use the probes to measure the pressure & temperature, but not to take the pressure reading until the temperature has stabilized, using the water bath as needed to maintain a constant temperature. Then change the volume of the piston and collect another measurement. Taking at least 2 measurements per volume level will help reduce experimental errors as well.
B) Here's a sample response: The student is not correct. As the pressure and volume change the temperature also changes. The gas temperature would need to be measured to verify that it has reached equilibrium with the water bath.
C) Taking the Pressure and Volume at a constant temperature, you could do two different options to determine if the gas is ideal.
- Plot a Pressure vs Volume graph. If it is linear, the gas is ideal
- Multiply Pressure & Volume. If the result is a constant (or relatively so) the gas is ideal (PV=nRT) D) Since the graph is a V vs T plot, we can rework the ideal gas law to find n.
V = (nR / P)*T The slope of the graph is nR/P where R and P are constants. So find the slope of the graph multiply it by P/R to find n.
E) Theoretically the volume of an ideal gas would be 0 at absolute zero. So extend the trendline backwards until it crosses the x-axis. This gives a rough estimate of absolute zero to be -300C. (You won't get credit for -273C because the graph isn't that precise).
Answers (Don't peek!👀)
A) The two other charges apply forces on the 500nC charge as shown
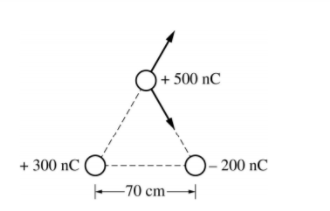
So we need to find the magnitude of each force using Coulomb's Law, then calculate the vertical and horizontal components of each force, sum them to get the components of the total force then determine the resultant.
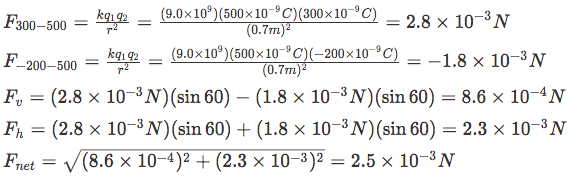
B) In triangles 3 and 4, the fields from each of the three spheres all point toward the center of the triangle or away from it. This is a symmetrical arrangement, so the net force is zero.
C) Potential is not a vector, so the potentials from each sphere simply add. Positive charges produce positive potential, and negative charges produce negative potential. The charges are equal, so all charges produce the same magnitude of potential at the center. V3 is caused by a net of 3 positive charges, V2 by 2 positives, V1 by 2 negatives, and V4 by 3 negatives. So the ranking should be V3 > V2 > V1 > V4.
<< Hide Menu
The Ultimate AP Physics 2 Exam Guide
7 min read•june 18, 2024
Peter Apps
Peter Apps
Introduction
A quick pep talk before we start
You've been (likely) prepping for months for this exam! 🤓 One test can never measure everything you've learned from studying physics. So please, don't let your AP scores define you!
Now that we've gotten that out of the way, let's focus a bit more on the actual exam.
Exam Logistics 🤔
The AP Physics 2 exam (just like almost every other exam) consists of 2 sections: Multiple Choice Questions (MCQs) and Free-Response Questions (FRQs).
In Section 1, you will have 90 minutes to answer 50 MCQs, whereas, in Section 2, you will have 90 minutes to answer 4 FRQs, which are listed below. Each section is weighted at 50% of your exam score, so it's important to prepare for both sections! 📚

Image Courtesy of AP Physics 2 Course & Exam Description
As far as trying to guess what topics will be on the exam, College Board has given us this rough estimate of how the questions will be broken down:

Image Courtesy of AP Physics 2 Course & Exam Description
Section 1: Multiple Choice Questions
This section mostly consists of (you guessed it) Multiple Choice Questions. You're more likely to be asked a question about modeling, argumentation, or mathematically calculating a value than you are to be asked questions about experimental methods, or data analysis.

Image Courtesy of AP Physics 2 Course & Exam Description
Strategies For the Multiple Choice Section 💪
- Read the question fully before trying to answer. There's often a tidbit in the image or description that is essential to correctly solve the problem. Don't just skim.
- It's ok to narrow down to 2 choices before guessing. CollegeBoard doesn't expect perfection and a 50% chance of getting the right answer is a lot better than leaving it blank.
- Reference Tables are your friend! Even if it's for something as simple as "What's the unit for charge?" It's a free resource so use it!
- Start with a question you know you'll get right! A little boost of confidence can be the difference between a very difficult test and one that's much easier.
- Watch your time! You've got about 90 seconds to answer each question. Don't feel bad about skipping a harder question and coming back to it if you have time. (Just make sure you don't leave any blanks on that answer sheet!)
Sample MC Questions
All of these questions can be found on the AP Physics 2 Course and Exam Description
Answers (Don't peek!👀)
- The correct answer is C
Kinetic Energy of a gas is proportional to the temperature (K = 3/2*kBT). You can calculate the temperature of the gas at each point using the Ideal Gas Law (PV = nRT). Plugging in for each of the 3 locations, we see that point A and C both have a temperature of (3P0V0) / (nR) while point B has a temperature of (P0V0) / (nR). Therefore A and C have the same temperature which is higher than point B.
- The correct answer is D
Pressure is defined as Force per unit Area. A higher pressure will result in a higher force being applied to the piston. Point C has 3 times the pressure as A & B so it will have a higher force.

Answer (Don't peek!👀)
The correct answer is C. The resistance of the cylinder is proportional to the length and inversely proportional to the cross-sectional area R = pL / A because the area of the cylinder depends on the square of the radius, doubling both the length and the radius will result in 1/2 as much resistance which should double the current.

Answer (Don't peek!👀)
The correct answer is B. Use Bernoulli's continuity equation


Answer (Don't peek!👀)
The correct answer is C. Bulbs X and Y are connected directly to the battery through the switch and therefore are controlled directly by the switch. Bulb Z relies on current passing through the capacitor to be lit. Initially the uncharged capacitor allows current to flow through it, until the voltage of the capacitor reaches the same voltage as the battery. When this happens there is no flow of current through bulb Z and it goes out.
Section 2: Free-Response Questions
So you've made it through the MCQ, now we've got 90 minutes each to tackle 4 FRQ's. Unlike the MCQs, we know the four question types. There will be an experimental design (12 points), qualitative/quantitative translation (12 points), paragraph argument (10 points), and one additional question (10 points). We're still focusing a lot on theoretical relationships, math routines, and arguments. This is also the section where derivations come into play.

Strategies for the FRQ 🤨
- Pace yourself! Look at the suggested times for each question and try to stick within those recommendations.
- Graphs and drawings are easy points. If you're crunched for time and have to choose between a graph section or a derivation, choose the graph one!
- Reference tables are there for a reason! Use them!
- Remember to BCDA your arguments (Bring in Physics concepts, Cite info from the problem, Draw the info and the physics together, and finally, answer the question)
- Know your vocab! (Check out the list below)
Vocab is fun!
- Calculate: Perform mathematical steps to arrive at a final answer, including algebraic expressions, properly substituted numbers, and correct labeling of units and significant figures. Also phrased as “What is?”
- Compare: Provide a description or explanation of similarities and/or differences.
- Derive: Perform a series of mathematical steps using equations or laws to arrive at a final answer.
- Describe: Provide the relevant characteristics of a specified topic.
- Determine: Make a decision or arrive at a conclusion after reasoning, observation, or applying mathematical routines (calculations).
- Estimate: Roughly calculate numerical quantities, values (greater than, equal to, less than), or signs (negative, positive) of quantities based on experimental evidence or provided data. When making estimations, showing steps in calculations are not required.
- Explain: Provide information about how or why a relationship, process, pattern, position, situation, or outcome occurs, using evidence and/or reasoning to support or qualify a claim. Explain “how” typically requires analyzing the relationship, process, pattern, position, situation, or outcome; whereas, explain “why” typically requires analysis of motivations or reasons for the relationship, process, pattern, position, situation, or outcome.
- Justify: Provide evidence to support, qualify, or defend a claim, and/or provide reasoning to explain how that evidence supports or qualifies the claim.
- Label: Provide labels indicating unit, scale, and/or components in a diagram, graph, model, or representation.
- Plot: Draw data points in a graph using a given scale or indicating the scale and units, demonstrating consistency between different types of representations.
- Sketch/Draw: Create a diagram, graph, representation, or model that illustrates or explains relationships or phenomena, demonstrating consistency between different types of representations. Labels may or may not be required.
- State/Indicate/Circle: Indicate or provide information about a specified topic, without elaboration or explanation. Also phrased as “What…?” or ”Would…?” interrogatory questions.
- Verify: Confirm that the conditions of a scientific definition, law, theorem, or test are met in order to explain why it applies in a given situation. Also, use empirical data, observations, tests, or experiments to prove, confirm, and/or justify a hypothesis.
Sample Free Response Questions
All of these questions can be found on the AP Physics 2 Course and Exam Description
Answers (Don't peek!👀)
A) There are a bunch of different procedures you could choose from. The big idea is to use the probes to measure the pressure & temperature, but not to take the pressure reading until the temperature has stabilized, using the water bath as needed to maintain a constant temperature. Then change the volume of the piston and collect another measurement. Taking at least 2 measurements per volume level will help reduce experimental errors as well.
B) Here's a sample response: The student is not correct. As the pressure and volume change the temperature also changes. The gas temperature would need to be measured to verify that it has reached equilibrium with the water bath.
C) Taking the Pressure and Volume at a constant temperature, you could do two different options to determine if the gas is ideal.
- Plot a Pressure vs Volume graph. If it is linear, the gas is ideal
- Multiply Pressure & Volume. If the result is a constant (or relatively so) the gas is ideal (PV=nRT) D) Since the graph is a V vs T plot, we can rework the ideal gas law to find n.
V = (nR / P)*T The slope of the graph is nR/P where R and P are constants. So find the slope of the graph multiply it by P/R to find n.
E) Theoretically the volume of an ideal gas would be 0 at absolute zero. So extend the trendline backwards until it crosses the x-axis. This gives a rough estimate of absolute zero to be -300C. (You won't get credit for -273C because the graph isn't that precise).
Answers (Don't peek!👀)
A) The two other charges apply forces on the 500nC charge as shown

So we need to find the magnitude of each force using Coulomb's Law, then calculate the vertical and horizontal components of each force, sum them to get the components of the total force then determine the resultant.
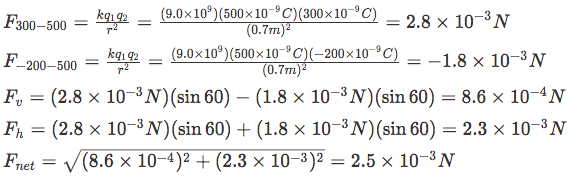
B) In triangles 3 and 4, the fields from each of the three spheres all point toward the center of the triangle or away from it. This is a symmetrical arrangement, so the net force is zero.
C) Potential is not a vector, so the potentials from each sphere simply add. Positive charges produce positive potential, and negative charges produce negative potential. The charges are equal, so all charges produce the same magnitude of potential at the center. V3 is caused by a net of 3 positive charges, V2 by 2 positives, V1 by 2 negatives, and V4 by 3 negatives. So the ranking should be V3 > V2 > V1 > V4.

© 2024 Fiveable Inc. All rights reserved.