Browse By Unit
Krish Gupta
Daniella Garcia-Loos
Krish Gupta
Daniella Garcia-Loos
2.1 Thermodynamic Systems 💤
What is Thermodynamics? 👀
Simply said, thermodynamics is the study of heat and energy. Temperature, kinetic energy, heat, work, and energy conservation are just some of the few topics we will learn about in this unit. There are two main parts to thermodynamics: classical and statistical 👓
Classical thermodynamics and mechanics looks at phenomena through a macroscopic lens. This type of thermodynamics is basically concerned with the big picture stuff. Equilibrium is a major topic that looks at the overall state of a process. In Physics 1, you mostly dealt with classical mechanics and largely overlooked atomic level explanations.
Statistical thermodynamics and mechanics looks at processes through a microscopic lens. This is your small picture stuff, like the atomic level. Entropy is a topic that we will learn about in this unit that is at the heart of statistical mechanics. The AP exam will not only ask you about big picture things and generic calculations but will also ask you to explain what is happening between the molecules and atoms in a scenario. That is the beauty of this course; We get the big picture and the small picture.
Efficiency brings statistical and classical thermodynamics together. Efficiency is the ratio between input energy and output energy. It basically tells you how much you got out of what you put in. In the real world, engineers are extremely concerned with efficiency. No machine is 100% efficient, yet every engineer’s goal is to maximize efficiency.
Here are some key differences between classical and statistical thermodynamics:
- Classical thermodynamics is based on the laws of thermodynamics and the concept of temperature, while statistical thermodynamics is based on the statistical behavior of particles in a system.
- Classical thermodynamics is a macroscopic approach that deals with the behavior of large systems, while statistical thermodynamics is a microscopic approach that deals with the behavior of individual particles in a system.
- Classical thermodynamics is concerned with macroscopic properties such as pressure, volume, and temperature, while statistical thermodynamics is concerned with the behavior of individual particles in a system and the statistical distribution of their energy states.
- Classical thermodynamics can be used to predict the behavior of a system based on macroscopic variables, while statistical thermodynamics is used to predict the behavior of a system based on the statistical behavior of its individual particles.
Overall, classical thermodynamics is a more general theory that can be applied to a wide range of systems, while statistical thermodynamics is a more specific theory that is used to understand the behavior of systems at the microscopic level.
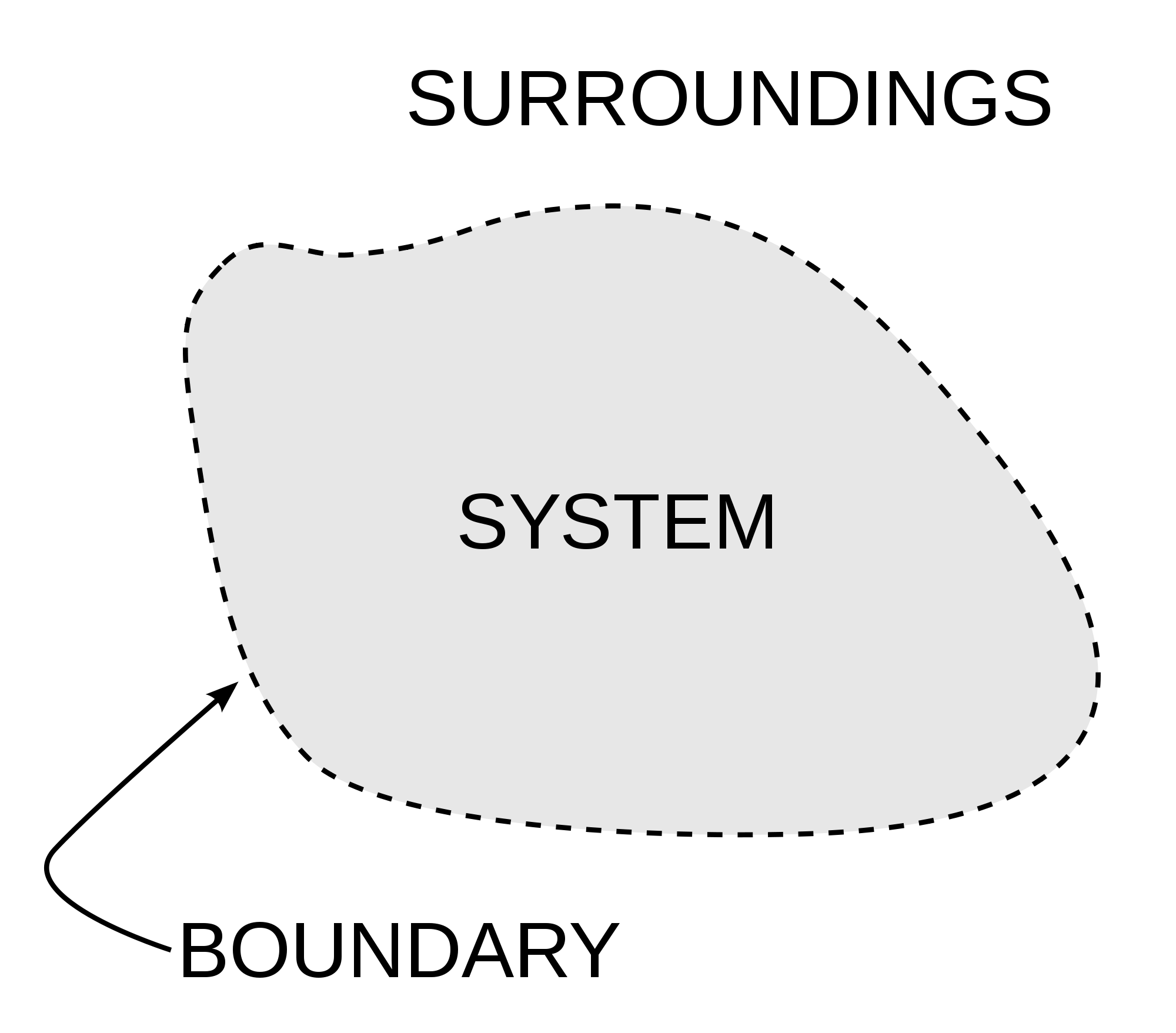
As the image shows Thermodynamics is all about interactions inside our system and between the system and its surrounding. Image Courtesy of Wikipedia.
In this unit, we will mostly be dealing with gases. You will get a broad overview of how machines and engines work. We will study Pressure-Volume (PV) diagrams. There will be a lot of math and theories involved. Lastly, we will put a specific focus on how refrigerators and heat pumps represent thermodynamics at work.
Object vs. System
You might remember this topic from the previous, unit but let’s review it quickly. An object is a defined collection of matter. A system is a collection of objects. If the system is not too complex and we only want approximate models, we can simply say that the system itself is an object (we did this all the time in Physics 1).
The Physics 2 writers really want you to know the difference between the words. The semantics of knowing the difference is really only important for Unit 1 and Unit 2. The math associated with this section is very straightforward, but you won’t believe how many people miss points simply because they put the wrong sign ⚠️
** ProTip: If you keep your positives and negatives in the right place and know what they mean, you will ace this section on the AP exam! 💕 **
Here are some key points about the difference between an object and a system in thermodynamics:
- An object in thermodynamics is a specific physical entity, such as a piece of metal or a container of gas. It has specific properties, such as mass, volume, and temperature, and these properties may change over time.
- A system in thermodynamics is a region of space that is being studied or analyzed. It is defined by a boundary that separates it from the rest of the universe.
- The properties of a system are determined by the interactions of its constituent parts, such as the atoms or molecules that make up the system.
- The behavior of a system is affected by its surroundings, which can exchange energy and matter with the system through the boundary.
- A system can be closed, meaning that it does not exchange matter with its surroundings, or open, meaning that it does exchange matter with its surroundings.
- The laws of thermodynamics apply to systems, not to objects. These laws describe how energy and matter are exchanged within a system and how this exchange affects the properties of the system.
Example Problem #1:
A container is divided into two parts by a partition. The left side of the container contains 1 mole of gas A, and the right side contains 1 mole of gas B. The partition is removed, and the gases mix and reach equilibrium. The total pressure in the container is 1 atmosphere.
- Draw a diagram of the initial state of the system, showing the two gases separated by the partition.
- Draw a diagram of the final state of the system, showing the two gases mixed together.
Example Problem #2:
Imagine that you are studying a solid object made up of atoms. You have a diagram that shows the arrangement of the atoms in the solid object.
- Using the diagram, draw a representation of the interactions between the atoms that make up the solid object.
- Explain how these interactions determine the properties of the solid object, such as its density, melting point, and strength.
- Consider a scenario in which the solid object is subjected to high temperatures. How might the interactions between the atoms change as the temperature increases? How would these changes affect the properties of the solid object?
<< Hide Menu
Krish Gupta
Daniella Garcia-Loos
Krish Gupta
Daniella Garcia-Loos
2.1 Thermodynamic Systems 💤
What is Thermodynamics? 👀
Simply said, thermodynamics is the study of heat and energy. Temperature, kinetic energy, heat, work, and energy conservation are just some of the few topics we will learn about in this unit. There are two main parts to thermodynamics: classical and statistical 👓
Classical thermodynamics and mechanics looks at phenomena through a macroscopic lens. This type of thermodynamics is basically concerned with the big picture stuff. Equilibrium is a major topic that looks at the overall state of a process. In Physics 1, you mostly dealt with classical mechanics and largely overlooked atomic level explanations.
Statistical thermodynamics and mechanics looks at processes through a microscopic lens. This is your small picture stuff, like the atomic level. Entropy is a topic that we will learn about in this unit that is at the heart of statistical mechanics. The AP exam will not only ask you about big picture things and generic calculations but will also ask you to explain what is happening between the molecules and atoms in a scenario. That is the beauty of this course; We get the big picture and the small picture.
Efficiency brings statistical and classical thermodynamics together. Efficiency is the ratio between input energy and output energy. It basically tells you how much you got out of what you put in. In the real world, engineers are extremely concerned with efficiency. No machine is 100% efficient, yet every engineer’s goal is to maximize efficiency.
Here are some key differences between classical and statistical thermodynamics:
- Classical thermodynamics is based on the laws of thermodynamics and the concept of temperature, while statistical thermodynamics is based on the statistical behavior of particles in a system.
- Classical thermodynamics is a macroscopic approach that deals with the behavior of large systems, while statistical thermodynamics is a microscopic approach that deals with the behavior of individual particles in a system.
- Classical thermodynamics is concerned with macroscopic properties such as pressure, volume, and temperature, while statistical thermodynamics is concerned with the behavior of individual particles in a system and the statistical distribution of their energy states.
- Classical thermodynamics can be used to predict the behavior of a system based on macroscopic variables, while statistical thermodynamics is used to predict the behavior of a system based on the statistical behavior of its individual particles.
Overall, classical thermodynamics is a more general theory that can be applied to a wide range of systems, while statistical thermodynamics is a more specific theory that is used to understand the behavior of systems at the microscopic level.

As the image shows Thermodynamics is all about interactions inside our system and between the system and its surrounding. Image Courtesy of Wikipedia.
In this unit, we will mostly be dealing with gases. You will get a broad overview of how machines and engines work. We will study Pressure-Volume (PV) diagrams. There will be a lot of math and theories involved. Lastly, we will put a specific focus on how refrigerators and heat pumps represent thermodynamics at work.
Object vs. System
You might remember this topic from the previous, unit but let’s review it quickly. An object is a defined collection of matter. A system is a collection of objects. If the system is not too complex and we only want approximate models, we can simply say that the system itself is an object (we did this all the time in Physics 1).
The Physics 2 writers really want you to know the difference between the words. The semantics of knowing the difference is really only important for Unit 1 and Unit 2. The math associated with this section is very straightforward, but you won’t believe how many people miss points simply because they put the wrong sign ⚠️
** ProTip: If you keep your positives and negatives in the right place and know what they mean, you will ace this section on the AP exam! 💕 **
Here are some key points about the difference between an object and a system in thermodynamics:
- An object in thermodynamics is a specific physical entity, such as a piece of metal or a container of gas. It has specific properties, such as mass, volume, and temperature, and these properties may change over time.
- A system in thermodynamics is a region of space that is being studied or analyzed. It is defined by a boundary that separates it from the rest of the universe.
- The properties of a system are determined by the interactions of its constituent parts, such as the atoms or molecules that make up the system.
- The behavior of a system is affected by its surroundings, which can exchange energy and matter with the system through the boundary.
- A system can be closed, meaning that it does not exchange matter with its surroundings, or open, meaning that it does exchange matter with its surroundings.
- The laws of thermodynamics apply to systems, not to objects. These laws describe how energy and matter are exchanged within a system and how this exchange affects the properties of the system.
Example Problem #1:
A container is divided into two parts by a partition. The left side of the container contains 1 mole of gas A, and the right side contains 1 mole of gas B. The partition is removed, and the gases mix and reach equilibrium. The total pressure in the container is 1 atmosphere.
- Draw a diagram of the initial state of the system, showing the two gases separated by the partition.
- Draw a diagram of the final state of the system, showing the two gases mixed together.
Example Problem #2:
Imagine that you are studying a solid object made up of atoms. You have a diagram that shows the arrangement of the atoms in the solid object.
- Using the diagram, draw a representation of the interactions between the atoms that make up the solid object.
- Explain how these interactions determine the properties of the solid object, such as its density, melting point, and strength.
- Consider a scenario in which the solid object is subjected to high temperatures. How might the interactions between the atoms change as the temperature increases? How would these changes affect the properties of the solid object?

© 2024 Fiveable Inc. All rights reserved.